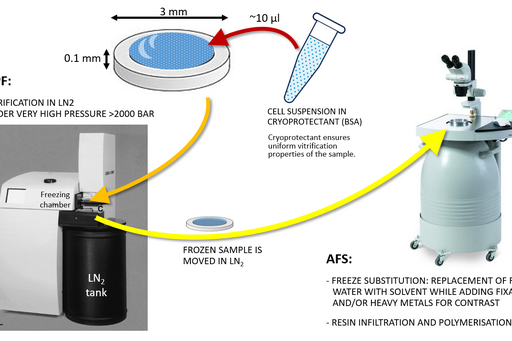
High pressure freezing and freeze substitution
To avoid the occurrence of artifacts, which can be introduced by chemical fixation, a sample can instead be vitrified under very high pressure in liquid nitrogen.
Chemical fixation introduces a number of artefacts due to factors such as slow diffusion, selective reactions between fixatives and cell components and osmolarity differences between fixative and specimen. Subsequently, proteins can cluster together as a result of crosslinking, membranes become “wobbly” and antigenicity is affected. To avoid or substantially minimize the occurrence of these artefacts, a sample can be vitrified in liquid nitrogen and under very high pressure (over 2000 bar) instead of being fixed chemically. This results in instant and simultaneous immobilization of all cell components without ice crystal formation and cell component disruption.
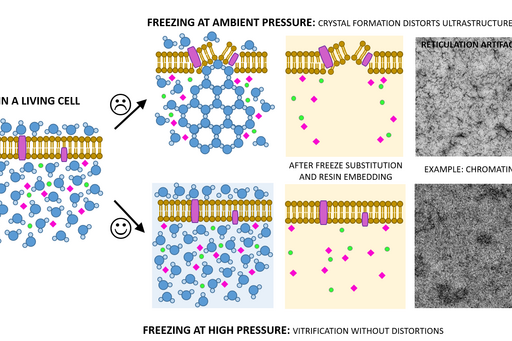
Sample size for high pressure freezing is limited as vitrification is effective only to the depth of 200 µm. Therefore a very small and thin piece of tissue or a very small volume (several microlitres) of cell suspension is required. A solution of cryoprotectant, most commonly an animal serum albumin, is used to surround the sample during the freezing to prevent drying out and ice crystal formation and optimise heat transfer.
Following the freezing, vitrified water is removed with solvent containing one or a combination of the following components to increase contrast and additionally fix the specimen: a heavy metal stain (most commonly uranyl acetate), formaldehyde, glutaraldehyde, osmium tetroxide, tannic acid. This process is known as freeze substitution. The solvent is finally replaced with liquid resin and cured at sub-zero temperature using UV light to avoid heat damage to epitopes.