Snabb metod för att följa proteinfosforylering
Unambiguous tracking of protein phosphorylation by fast high-resolution FOSY NMR
Område
Naturvetenskap &
IT
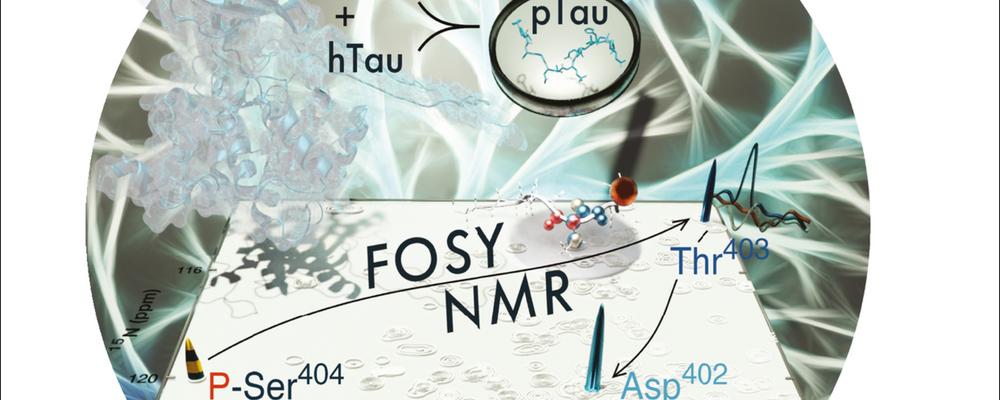
Unambiguous tracking of protein phosphorylation by fast high-resolution FOSY NMR
Dysregulation of post-translational modifications (PTMs) like phosphorylation is often involved in disease. NMR has the capabilities to elucidate the exact loci and time courses of PTMs at atomic resolution and near-physiological condition, but requires signal assignment to individual atoms. Conventional NMR methods applicable for such questions are based on tedious global signal assignment that require weeks for the experiment and analysis and may often fail, as for large intrinsically disordered proteins (IDPs). Here an international team with Björn Burmann and lead by Vladislav Orekhov present a robust alternative to rapidly obtain needed assignment of PTM sites, based on the novel concept of FOcused SpectroscopY (FOSY) experiments that combine the ultrahigh signal dispersion of up to six- or seven-dimensional (6D, 7D) spectra with the sensitivity, speed, and simplicity of 2D spectra. As a showcase application for the proposed FOSY approach to monitor PTMs in IDPs, in only couple of hours, we identify two phosphorylated residues in vitro by proline-dependent glycogen synthase kinase 3 beta (GSK3β) in the 441-residue long human hTau40 protein, which comprises 80 serines and threonines as potential phosphorylation sites. Abnormal hTau40 hyper-phosphorylation is directly linked to dysregulation and, possibly, aggregation that characterises neurodegenerative tauopathies such as Alzheimer’s disease.
Dmitry M. Lesovoy, Panagiota S. Georgoulia, Tammo Diercks, Irena Matečko-Burmann, Björn M. Burmann, Eduard V. Bocharov, Wolfgang Bermel, and Vladislav Y. Orekhov
Angew. Chem. Int. Ed. 2021, 60, 1 – 6, doi.org/10.1002/anie.202102758