- Home
- Research
- Find research
- Heithem Ben Amara: Biodegradable implants and tissue healing
Heithem Ben Amara: Biodegradable implants and tissue healing
Long-term effects of biodegradable magnesium implants are well studied, but the immediate reactions in the body are still a mystery. In his doctoral project, Heithem Ben Amara delves into the initial responses of soft tissue and bone to magnesium implants and how they heal afterward.
HEITHEM BEN AMARA
Dissertation Defense: November 17, 2023
Doctoral Thesis: Biodegradable magnesium implants, immunomodulation, and tissue repair/regeneration
Research Area: Biomaterials
Sahlgrenska Academy, The Institute of Clinical Sciences
What is the background of your doctoral project?
“For over one hundred years, we have used metallic implants, like stainless steel or titanium, to fix broken bones. Once they have fulfilled their task, they are often removed. But now, there is a trend towards using other metallic implants made of magnesium that degrade, releasing ions and gas,” says Heithem Ben Amara, a dentist from Tunisia who came to Gothenburg for his PhD-studies after earning a master’s degree in Periodontology in South Korea.
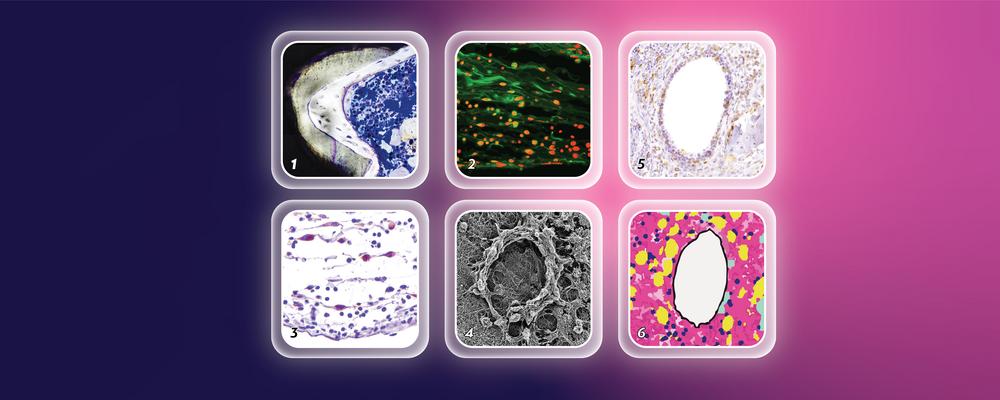
“While long-term effects of magnesium implants are well studied, the immediate reactions in the body are still a mystery. Some believe these implants reduce inflammation, but this belief is challenged by the temporary inflammation seen near them, which often occurs around bubbles resulting from gas release by the degrading implant in patients.”
Magnesium vs titanium
What is your research about in brief?
“We wanted to see how the body’s soft tissue and bones initially respond when magnesium implants are used, and how they heal afterward. Compared to the state-of-the art non nondegradable titanium, we tried two different types of magnesium, pure and alloyed, to see if they make a difference in the bone response. The alloyed magnesium is already in use in patients,” says Heithem Ben Amara.
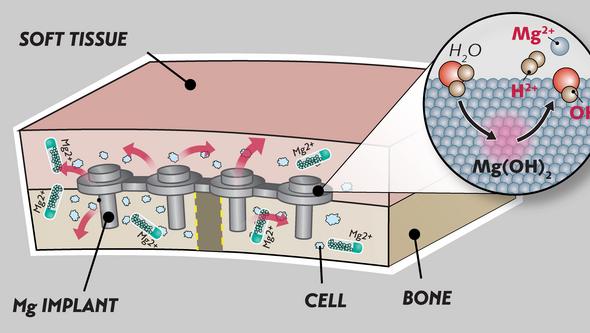
Joint efforts across Europe
He has worked on his doctoral project within an international training network called “MgSafe”, which is a part of the European research program Marie Skłodowska-Curie Actions.
“Within ‘MgSafe’, we joined our efforts with twelve institutions from all over Europe that hosted fifteen PhD students co-working to improve the imaging and the understanding of the biological response to magnesium implants,” says Heithem Ben Amara, and continues:
“The Department of Biomaterials at the University of Gothenburg is known for its groundbreaking work with titanium implants. So, we used well-characterized in vivo models and a toolbox of techniques previously employed here to zoom in on the cellular reactions to magnesium implants.”
What are your most significant results?
“Magnesium helps soft tissue and bone heal better than the commonly used titanium. It reduces the fibrotic response in soft tissue and increases bone formation at the implant surface. Contrary to popular belief, this does not happen after a reduced initial inflammation, but a transient surge in inflammation.”
Different effects on bone healing
Heithem Ben Amara’s thesis also shows that different types of magnesium can affect bone healing differently.
“Unlike titanium and clinical-grade alloyed magnesium, pure magnesium leads to previously unreported fat accumulation in the bone marrow and immature bone formation at the implant surface, possibly due to persistent low-grade inflammation. We think that the faster degradation of pure magnesium compared to alloyed magnesium in bone might play a role.”
What practical applications can these results have?
“I think we should monitor magnesium implant patients more closely, especially their bone marrow and the function of the new bone that forms at the surface of these implants.”
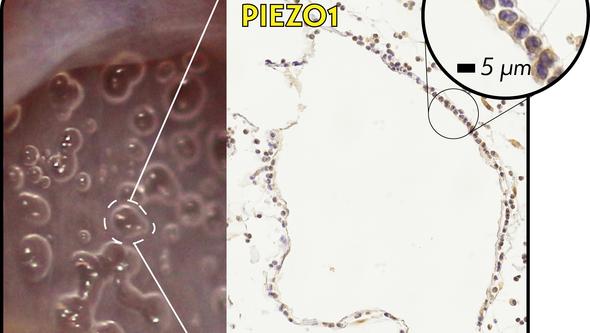
“Mysterious gas bubbles”
“We also had a closer look into those mysterious gas bubbles around the implants. There is debate about how they affect healing, so we studied the response of cells near these bubbles,” says Heithem Ben Amara, and adds:
“We found that these cells have an inflammatory behavior. They apparently ‘feel’ the pressure by the gas bubbles as they express a mechanosensitive protein called PIEZO1. These inflammatory and mechanosensitive effects by the bubbles might play a part in how tissues heal around magnesium implants.”
Text: Jakob Lundberg
