
- What is Patient and Public Involvement (PPI) in research?
- Why involve patients and the public in research?
- Which persons can be involved?
- How does one involve patients and the public in research?
- How does one recruit PPI representatives?
- At which stage in a research project can patients and the public be involved?
- Examples of PPI in research
- Links:
- Articles
- Web resources
- Patient and public involvement (PPI) in research workshop material
Patient and Public Involvement (PPI) in research – how, when and why
Involving patients and the public can improve the relevance and quality of research projects. Here are some tips on how, when and why patients and the public can be involved in research.
What is Patient and Public Involvement (PPI) in research?
Public involvement in research means research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. This includes, for example:
- working with research funders to prioritise research;
- offering advice as members of a project steering group;
- commenting on and developing research materials;
- undertaking interviews with research participants.
This is the definition used by British government funded advisory group INVOLVE. By the ‘public’ INVOLVE means patients, potential patients, carers and people who use health and social care services as well as people from organisations that represent people who use services.
There is an important distinction to be made between the perspectives of the public and the perspectives of people who have a professional role in health and social care services, even if these professionals often also have experience of being patients and/or carers themselves.
Why involve patients and the public in research?
The exact reasons why one should involve patients and the public in research will be specific to each project, but here are some general reasons:
Get different perspectives. Patients and the public will be able to provide different perspectives from those of the professional researchers. They can contribute with their own expertise and lived experience of for example illness and the impact of drugs and treatments.
Improve research quality and relevance. Involving patients and the public will improve research quality and relevance. This can be compared with the fact that virtually all commercial companies now start any product or service development process by consulting the end user.
It’s the right thing to do. A lot of research is funded by public funding – therefore involving the public is the right thing to do.
Major scientific publications encourage PPI. For example The British Medical Journal supports what they describe as an international movement towards active involvement of patients and the public as co-producers of research. They require authors submitting research papers to include a Patient and Public Involvement Statement.
Which persons can be involved?
Choosing PPI representatives for your steering group. A good start can be to involve a couple of patient and public involvement representatives, or PPI representatives for short, in a research group or research department steering group. The speciality of the research group or department will determine which PPI representatives will be best suited. Perhaps the study area is a disease or a syndrome? Persons with a comprehensive own experience or experience as a carer/family member of that condition should be able to provide the right input.
Depending on the speciality, perhaps some persons who are patients at a relevant local clinic or members of a national or local patient organisation, which represents this patient group can be the right choice. We at GPCC decided to have a very mixed group of persons who together bring a long and varied experience of Swedish healthcare in our Person Council for Patients and Relatives/Carers, as this suited us best.
Choosing PPI representatives for your specific research project. If the project focuses on a particular patient group, some representatives from that patient group and/or family representatives should be suitable. If there are already PPI representatives in the steering group, they will probably be able to advice on where to find persons with the right experience.
How does one involve patients and the public in research?
Before approaching any potential PPI representatives, it is important to think through what the purpose of this involvement is and what it will look like.
Outline and document the framework. It is a good idea to draft some simple plain language documents outlining things like aim and objectives, definition of roles and a tariff for payment for time and travel expenses. It is also important to get the PPI representatives’ input on these documents once they have been hired, and possibly to revise them after that.
It may be a good idea to include this information as well as costs for PPI in the research proposal budget. HR or the finance department may want to be involved in the creation of this material, and may be able to offer advice.
Respect the PPI representatives time. Be very clear about how much time the PPI representatives are expected to devote, and provide times, dates and locations as early as possible. This should be reviewed together with the PPI reps, and may need to be altered depending on their circumstances and availability.
Make them partners in Research. It is important that the researchers are aware of aspects of accessibility to accommodate the PPI representatives. This of course involves any physical needs they may have due to disabilities or impairments. But it is also very important to use inclusive language whenever the PPI reps take part, and avoid or explain any jargon or acronyms.
Also consider the power difference between the PPI reps and the researchers. It is not unusual for PPI reps to feel some degree of intimidation, conscious or not, as academics, and especially professors and doctors have a high status in our society. Therefore it is necessary to ensure that the researchers make it very clear that they value the PPI reps expertise, for example in the form of lives experience, and, if possible, always have two PPI reps as they may feel more confident in numbers.
How does one recruit PPI representatives?
When it has been established which type of PPI representatives one would like to involve, the next step is to work out how to find and recruit them. If one is looking for persons who are patients at a local clinic, one might consider putting up notices in the clinic waiting room, or advertising in a local newspaper. If one is looking for members of a local or national patient organisation, one should contact them. Some organisations have a group of patients and/or family/carers ready to take on this sort of task, and may already have provided them with training in how to be a patient research partner. There are also a myriad of social media forums for various illnesses and conditions, such as Facebook groups, which are often very welcoming to researchers interested in them.
At which stage in a research project can patients and the public be involved?
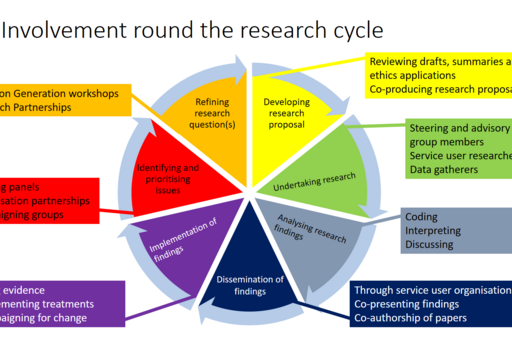
Defining and prioritising issues. Involving patients and the public to define and prioritise issues, which can lead to research projects means one will be more certain that the issues will be relevant to the end user. Tips on how to do this can be found on The James Lind Alliance website. PPI representatives can also be involved in commenting on applications for research funding.
Refining the research questions. PPI representatives can contribute with their different perspectives and lived experience in refining the research question. They can pinpoint what really matters to them, which maybe something different to what the researchers had thought. For example the main thing bothering a patient group could be fatigue rather than pain. If there are already PPI representatives recruited, they can be involved. If a bigger group needs to be consulted for this type of exercise, they can be so, for example in a focus group style workshop or using an on-line questionnaire .
Developing the research proposal. PPI representatives can contribute by co-producing part of or the whole research proposal text. They can also be involved in reviewing drafts. This ensures that the text is relevant and comprehensible from a patient perspective. Many funders now require and reward this.
PPI reps can be involved in the development of any documents for data gathering such as patient questionnaires as well as any accompanying documents like cover and consent letters. This can not only make documents more relevant and comprehensible to the study participants, it can also be advantageous in for example an ethics application process.
Undertaking the research. There are a number of ways in which PPI representatives can be involved in the undertaking of the research. These will be determined by the nature of each project, but here are some general examples. PPI representatives who are members of the project steering group can comment on the progress and development of the project. PPI representatives can also be involved in gathering data, for example as interviewers with a peer perspective.
Analysing the research findings. The PPI representatives may be able to contribute with different perspectives in comprehending and interpreting the research data. For example if it is unclear to the researchers what the study subjects are referring to, the PPI representatives may understand, as they have similar lived experience. They may also be able to bring suggestions on how to analyse and code the data.
Dissemination of the findings. Clearly outlining the involvement of the PPI representatives’ contribution in the research project or involving them as co-authors in academic publications and other reports and documents emanating from it is a good idea for many reasons. Not only does it give credit to the PPI representatives, it also shows readers that the research team is progressive. Many publishers of research papers are now moving towards working more with PPI themselves.
PPI representatives can also be useful in helping to disseminate the research findings. They may be better at explaining things in plain language, and they may be able to contribute with a personal story, thus adding depth to the findings. They may also have a relevant personal and organisational network, where the findings can be disseminated.
Implementation of findings. Today’s researchers are increasingly expected to take responsibility for the utilisation of their research, and there are ways in which the PPI representatives can be involved. They can for example spearhead the use of a new treatment or campaign to have new treatments, methods or other changes implemented.
Examples of PPI in research
Links:
Articles
Clarifying the roles of patients in research BMJ Editorial by Professor Britten et al. 2018.
Public involvement in health research: what does ‘good’ look like in practice? K. Liabo et al. 2020.
Web resources
Patient and next-of-kin collaboration for better research and healthcare (biobanksverige.se)
University of the West of England guidance document: Evaluating public involvement in research
INVOLVE UK National Standards for Public Involvement.
James Lind Alliance Priority Setting Partnerships.
3 short webinars by INVOLVE –Patient and public involvement in research; what, why and how.
7 top tips for involving patients in your research from cancer Research UK.
Filmed patient voices Health Talk online.
Patient and public involvement (PPI) in research workshop material
About this web page
This page was developed by Jeanette Tenggren Durkan in collaboration with Associate professor Charles Taft, Professor Eva Jakobsson Ung, the GPCC Person Council for Patients and relatives and The GPCC Steering Group. The content mainly comes from INVOLVE and Professor Nicky Britten and Dr Kath Maguire from The University of Exeter. Professor Britten was a Visiting Professor to GPCC during 2016-2020, and continues to be a Scientific Advisor to the Centre. Professor Britten and Dr Maguire held a well attended Sahlgrenska Academy Seminar and workshop on PPI in 2014. Material from that workshop is available under links on this web page.
Examples of reviews of the research area
"Defining Patient Engagement in Research: Results of a Systematic Review and Analysis: Report of the ISPOR Patient-Centered Special Interest Group" from 2020
"Frameworks for supporting patient and public involvement in research: Systematic review and co‐design pilot" from 2019
"Patient involvement in preparing health research peer-reviewed publications or results summaries: a systematic review and evidence-based recommendations" from 2020.