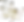Fredrik Sterky Group
Short description
Fredrik is an assistant professor at the Department of Laboratory Medicine and a resident physician in clinical chemistry with research time within Wallenberg Centre.
About Fredrik Sterky Group
Research Summary
Our daily thoughts and actions depend on the flow of information through neural circuits, mediated by the specialized cell-cell contacts called synapses that relay signals between contacting neurons. Impaired synaptic function underlies several neurodevelopmental and psychiatric disorders e.g. autism and schizophrenia, and synapse pathology and loss are also hallmark features of common neurodegenerative diseases. The formation and maturation of synaptic connections depend on interactions between synaptic cell adhesion proteins that span the synaptic cleft to form a physical bridge between contacting neurons. A well-known example is that of pre-synaptic neurexins and its interaction with diverse post-synaptic partners, which shape the properties of a synapse by defining its molecular architecture. Mutations in the gene encoding neurexin-1 have also repeatedly been found to confer genetic risk for autism and schizophrenia.
We are interested in the protein-protein and protein-glycan interactions, such as that of neurexins and their ligands, sculpt synaptic connections. The aim is to expand our understanding of the fundamental building blocks of our brains, and thereby also better understand the diseases that result from their dysfunction. More specifically, we aim to:
(1) Understand the function of carbonic anhydrase-related proteins and their possible role in regulating synaptic cell-adhesion complexes.
The carbonic anhydrase-related proteins are structural homologues to the classical carbonic anhydrase enzymes, but have lost their enzymatic activity during evolution. Yet, they are highly conserved, implying they have other important functions in our brains, where they are almost exclusively expressed. We have previously found that the carbonic anhydrase-related proteins CA10 and CA11 can bind to the neurexin family of presynaptic cell adhesion molecules (Fig. 1). The binding of CA10 to neurexins can, at least under some conditions, regulate the surface-levels of specific neurexin isoforms (Sterky et al., PNAS 2017). Yet, the underlying mechanism and normal role of CA10 and CA11 in the brain remains unknown. We are currently addressing these fundamental issues using a combination of mouse genetics, proteomics and protein biochemistry. This project serves to reveal the function of two conserved proteins in our brains and also provide a means to understand the post-translational regulation of the neurexin-complex.
(2) Study mechanisms of synapse development.
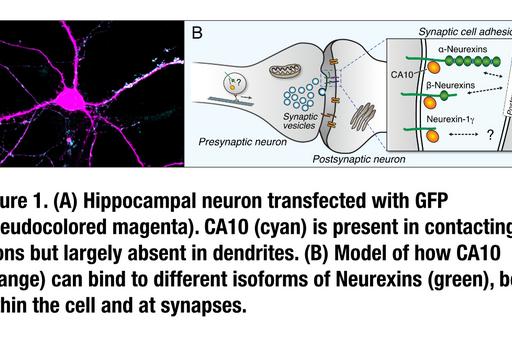
In addition to neurexins, several other families of cell surface receptors have the ability to induce pre- or post-synapse formation onto contacting neurons. While it is generally believed that clustering of these receptors at sites of contact results in the recruitment of intracellular scaffold proteins that assemble additional synaptic components, the intracellular pathways and signals that links receptor binding to synapse formation are not well understood. We have developed a reduced, in vitro system based on human induced neurons (‘iN cells’, Zhang et al., Neuron 2013, 78: 785–798) (Fig. 2) that we utilize in combination with gene-editing and protein biochemistry to address mechanisms of pre-synapse assembly.
(3) Investigate mechanisms of rare genetic disease by translational modelling in human neurons
Disease that result from deleterious variants in a single gene are individually rare, but collectively not uncommon. Next-generation sequencing technologies (e.g. whole-exome/-genome sequencing) have revolutionized their diagnostics, and to date more than 5000 rare genetic diseases have been described. A majority affect the central nervous system, which often cause debilitating symptoms. Most of these conditions are not well understood and specific therapies are lacking. However, in cases where the underlying cause (the genetic variant) has been identified, understanding its consequences on cellular physiology may suggest therapeutic options. Studies of rare genetic diseases may also lead to insights into normal physiology and the underlying biology of idiopathic cases with the same symptomatology.
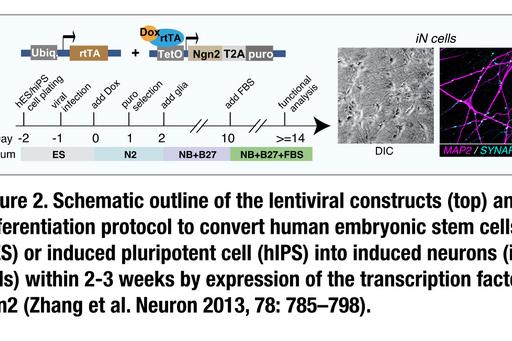
We focus on patients with developmental delay, intellectual disabilities and/or moderate-severe autism spectrum disorder for which clinical genetic sequencing has identified a likely causative genetic variant. By investigating either patient-derived cells or cells from healthy donors that have been gene-edited to carry disease-relevant genetic variants (Fig. 3) we aim to identify cellular disease mechanisms with the ultimate goal to also identify strategies to normalize cellular physiology.

Contact Information
Fredrik Sterky
Group Members
Debora Kaminski
Lab manager/technician
Berta Marcó
PhD Student
Angela Molinaro
Postdoc
Sanhita Mitra
Postdoc
Key Publications
Bi-allelic VPS16 variants limit HOPS/CORVET levels and cause a mucopolysaccharidosis-like disease. Sofou K, Meier K, Sanderson LE, Kaminski D, Montoliu-Gaya L, Samuelsson E, Blomqvist M, Agholme L, Gärtner J, Mühlhausen C, Darin N, Barakat TS, Schlotawa L, van Ham T, Asin Cayuela J, Sterky FH. EMBO Mol Med. 2021 May 7;13(5):e13376. doi: 10.15252/emmm.202013376
CA10 regulates neurexin heparan sulfate addition via a direct binding in the secretory pathway.
Montoliu-Gaya L, Tietze D, Kaminski D, Mirgorodskaya E, Tietze AA, Sterky FH.
EMBO Rep. 2021 Apr 7;22(4):e51349. doi: 10.15252/embr.202051349
Jennions E, Hedberg-Oldfors C, Berglund AK, Kollberg G, Törnhage CJ, Eklund EA, Oldfors A, Verloo P, Vanlander AV, De Meirleir L, Seneca S, Sterky FH†, Darin N (2019) TANGO2 deficiency as a cause of neurodevelopmental delay with indirect effects on mitochondrial energy metabolism. Journal of Inherited Metabolic Disease. 42:898-908.
Sterky FH†, Trotter JH, Lee SJ, Recktenwald CV, Du X, Zhou B, Zhou P, Schwenk J, Fakler B, Südhof TC (2017) Carbonic anhydrase-related protein CA10 is an evolutionarily conserved pan-neurexin ligand. Proceedings of the National Academy of Sciences USA 114:E1253-E1262.
Lee SJ, Wei M, Zhang C, Maxeiner S, Pak C, Calado Botelho S, Trotter J, Sterky FH, Südhof TC (2017) Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. Journal of Neuroscience 37:1062-1080.
Sterky FH, Lee S, Wibom R, Olson L, Larsson N-G (2011) Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proceedings of the National Academy of Sciences USA 108:12937–12942.
Wibom R, Lasorsa FM, Töhönen V, Barbaro M, Sterky FH, Kucinski T, Naess K, Jonsson M, Pierri CL, Palmieri F, Wedell A (2009) AGC1 deficiency associated with global cerebral hypomyelination. New England Journal of Medicine 361:489–495.
Grants and Awards
External grants
- The Swedish Research Council (Vetenskapsrådet) Establishment Grant
- Jeansson Foundations
Institutional support
- Wallenberg Centre for Molecular and Translational Medicine
- Sahlgrenska Academy (Returnee Grant)
- ALF Västra Götaland Region
Previous grants
- The Swedish Research Council (Vetenskapsrådet) International Postdoc Grant (2013-2016)
- The Sweden-America Foundation
- The Swedish Royal Academy of Sciences PE Lindahl Foundation