
3D bioprinting and stem cells in plastic surgery
Short description
Stem cells are found in every bodily organ, in several forms. Their function is to ensure that a certain type of tissue can be continuously replaced during our lives, and to assist healing of an injury to the tissue concerned.
A basic feature of stem cells is that they can be stimulated to develop into several different tissue types. This makes it possible to obtain tissues, such as cartilage or skin, that are used in a plastic surgery clinic.
All regenerative medicine needs to have a source to produce materials from, and we have equipment that can extract large quantities of patient-specific fat stem cells from individuals’ fat obtained by liposuction. We have induced these stem cells to turn into cartilage.
General background and objectives
Stem cells are found in every bodily organ, in several forms. Their function is to ensure that a certain type of tissue can be continuously replaced during our lives, and to assist healing of an injury to the tissue concerned.
The largest and most readily available source of stem cells is adipose (fatty) tissue, from which adipocyte stem cells are derived. These can be induced to form several different tissue types, such as cartilage. In a plastic surgery clinic, there is an ample supply of adipose tissue, for example after liposuction, and adipose tissue is thus an easily accessible source from which stem cells can be extracted.
A basic feature of stem cells is that they can be stimulated to develop into several different tissue types.
Stem cells can either themselves develop into a particular cell type or exert their effect by stimulating the growth of other, specific, cells for growth. This is a fundamental principle that helps to produce new tissue. It thereby becomes possible to obtain tissues, such as cartilage or skin, that are used in a plastic surgery clinic. The overall idea in this regard is to produce stem cells from the patient's own adipose tissue.
All regenerative medicine needs to have a source to obtain material from, and since the previous application we have acquired equipment and been able to successfully extract large quantities of patient-specific adipocyte stem cells. We have stimulated these stem cells to turn into cartilage. All in all, we thus have an almost inexhaustible source of patient-specific stem cells that can be used to create patient-specific tissues, and also to influence immune responses.
Background
One overall problem in all reconstructive plastic surgery is lack of tissue. Traditional techniques include such methods as removing cartilage from ribs, for example, to use in reconstructing ears, expanding skin mechanically, transplanting skin from one body area to another, and so on.
New technology means that cultured tissue may become an option for remedying the lack of suitable tissue.
Another development for creating tissue is 3D bioprinting, in which a 3D printer, loaded with bioinks and living cells, prints out the required tissue. Technological development has come a long way, with bioinks that have extremely specialized properties. The inks are shear-thinning, which means that they are relatively viscous in their resting state while becoming more fluid when they are subjected to pressure in the printer needle. The inks provide an environment in which cells thrive and grow. The components of the inks can be made resorbable or nonresorbable, depending on what the operator wants to achieve. The maximum resolution of the best printers is in the order of 10 micrometers — that is, in principle, the diameter of one cell.
Analogously to the fact that tissues are in short supply, the specific cells (such as cartilage cells) that are mixed in the ink are also scarce. The established method to remedy this is to mix specific cells with stem cells. The stem cells can either themselves develop into the desired cell type or stimulate growth of the specific cells that have been added.
For some years, a collaboration has been established between the University of Gothenburg Department of Plastic Surgery and Chalmers’ BBV Laboratory. We have conducted a series of experiments in which we 3D-bioprinted cartilage and skin. Using stem cells in combination with cartilage cells, we showed that the stem cells boost cartilage cells’ growth. In the past, we have had to rely on purchased, cultured stem cells from either bone marrow or fat. These commercial stem cells are not, of course, individual-specific; moreover, they are difficult to grow, availability can be a limiting factor, and the stem cells’ quality is difficult to control. Now, with our own ability to extract stem cells from fat, we have paved the way for almost unlimited access to fat stem cells. The research group's overall goal is to create tissue that is specific to a given patient. Then, if the 3D-bioprinted tissue is of sufficiently high quality, we get no immunological rejection problems when the tissue is inserted in the patient.
3D-bioprinted cartilage
There are three kinds of cartilage in the body:
- Hyaline cartilage, in weight-bearing joints, contains about 80% water, relatively few cartilage cells (chondrocytes), and thin Type II collagen fibrils.
- Fibrous cartilage, in the intervertebral discs, contains thicker fibrils of Type II collagen.
- Elastic cartilage, in the outer ears, contains a relatively high density of chondrocytes, and fibers of the protein elastin.
Cartilage lacks blood vessels and nerves, and has a very limited ability to heal. If cartilage is damaged, there is a loss of shape and function.
For reconstruction of the outer ear, the following technique is used today, Rib cartilage is taken, sculpted into the shape of an outer ear, and put in place under the skin on the side of the skull. With several consecutive operations, an outer ear is finally obtained. The whole process is characterized by the many hours’ surgery required. It involves considerable pain in the source site, and the results are often suboptimal.
3D bioprinting of cartilage means that cartilage can be printed in the desired shape with high resolution. In the long run, the goal is to be able to print an outer ear that is covered with skin and can be transplanted. The idea is that the patient gains a very lifelike ear, built out of the patient's own cells, while the painful, protracted surgical process is avoided.
Plan of work
3D-bioprinted constructs are printed with a bioink composed of nanocellulose. The construct contains either cartilage cells only or a combination of human cartilage cells and human stem cells, the latter being taken from bone marrow or fat. The construct is implanted in a nude mouse and removed after 30 and 60 days, respectively. Long-term experiments, in which we let the construct remain for as long as the animal's lifespan allows, show that the construct is not converted into bone and that the cartilage cells’ growth is not uncontrolled.
The morphology of the constructs is analyzed under a microscope.
FISH (fluorescence in situ hybridization) analysis is used to determine the origin of the visible cells. Glycosaminoglycans are detected through staining with Alcian blue and Safranin-O. Type II collagen is detected immunohistochemically.
The cartilage cells’ growth is analyzed by counting of all nucleus-bearing cells that simultaneously have cytoplasm containing glycosaminoglycan.
Preliminary results
The cartilage cells survive the printing process and grow into the construct in vivo. Adding stem cells from bone marrow increases cell growth, but the stem cells themselves do not turn into cartilage cells. Instead, the mechanism of action is that they stimulate the cartilage cells involved in the printing process. The construct retains excellent mechanical properties after 30 and 60 days in vivo respectively. Measurements of mechanical properties after 10 months in vivo show that constructs containing cartilage cells are mechanically superior to constructs without them. Macroscopically, the constructs are reminiscent of cartilage and are white, shiny, durable, and easy to handle.
The cartilage cells produce glycosaminoglycans and Type II collagen — that is, they do what they are expected to do.
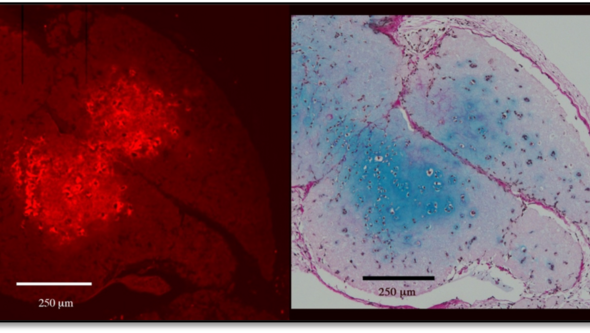
Figure 1. The red staining for Type II collagen, above left, and the blue staining for glycosaminoglycans, right, are visible. The incisions are consecutive. Note how the staining for each subject is most intense over exactly the same area, which indicates that it is the actual cartilage cells that are producing both substances.
FISH shows that it is the cartilage cells that have proliferated, while the stem cells have disappeared.
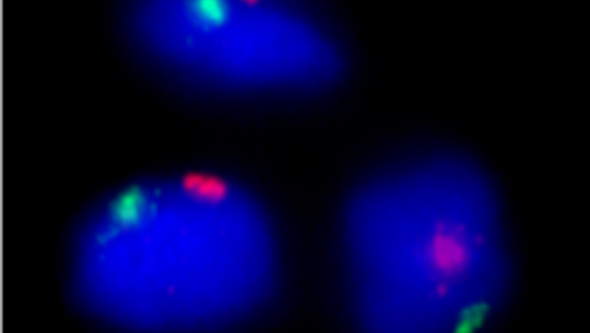
Figure 2. Above, we see how the cells are positive for both human chromosomes, X and Y: these are male cells from the cartilage donor that have grown. The female stem cells are not visible.
Skin that is transplanted onto a 3D-bioprinted piece of cartilage grows, adheres firmly, and heals edge to edge with the animal's own skin. The printed pieces thus have the potential to be included as the base for a more complex construction.
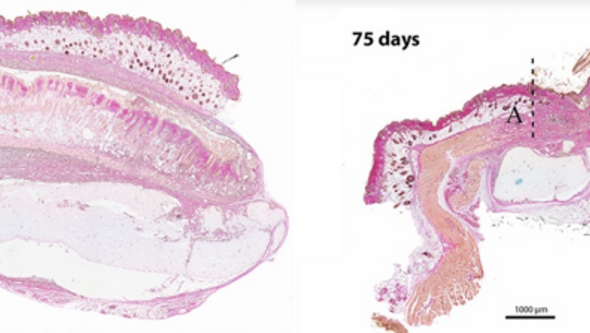
Figure 3. Above left, we see a full-thickness skin graft that has become attached to the base of 3D-bioprinted cartilage. The animal's own skin lies as a protective layer over the combined cartilage and skin construction. To the right, we see how the animal's own skin has been sewn edge to edge with the graft, which is on the 3D-printed piece of cartilage. The seamless junction between the animal’s own skin and the graft is at A, while B is the 3D-printed cartilage and C is the skin graft.
Next stage
The next stage in the experiment series is to change the source of the stem cells. In the past, we have used stem cells from bone marrow, but these are difficult to obtain and, to get a sufficient number, must be purchased and then grown. Thanks to the funds we received from IngaBritt and Arne Lundberg's Research Foundation in 2018, equipment was procured that allows us to extract fat stem cells from adipose tissue that we get when liposuction of plastic surgery patients. In addition to stem cells, we also get vascular (endothelial) cells and ‘progenitor cells’, which compose the walls of blood vessels. If we choose not to sort out the different fractions, the mixture of cells is called the stromal vascular fraction (SVF). Copious stem cells are used in the preparations. The cells are sorted and counted in a flow cytometer, using combinations of antibodies that attach to the various cell types.

Figure 4. The image above shows the results of our own flow-cytometric analyses of the cell contents in stromal vascular fraction (SVF) prepared with the Cytori process. Viability tests, using the 7-AAD exclusion method, detect some 97% of living cells, and of these roughly 3% consist of adipocyte-derived stem cells (ASCs) with the phenotype CD73+CD90+CD31-CD45- (green cloud)
‘We have stimulated these stem cells to be transformed into cartilage. All in all, this means that we now have an almost inexhaustible source of stem cells, which in turn can form different tissues. These cells are specific to the patient the fat comes from, which means that we can generate specific cells and tissues from virtually all the patients, since they all have adipose tissue that can be used.’
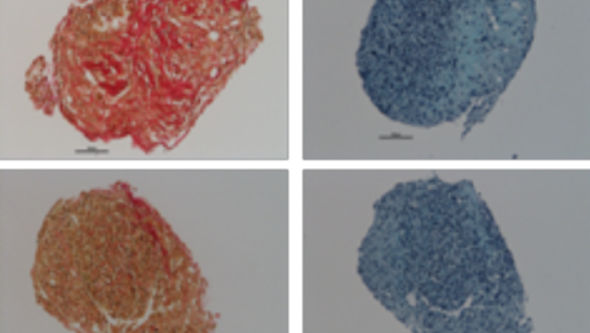
Figure 5. Differentiation of ASC (top) and SVF (bottom) from the same sensor. Staining with Safranin O (red) and Alcian Blue (blue) shows cartilage differentiation after approximately 3 weeks of in vitro stimulation in pellet formation. We see that the results are very similar irrespective of whether the origin is SVF or the ASCs are sorted out first. The results indicate that we can create cartilage from fat specifically for every patient.
Either the cells can be included directly in the printing process or they can be grown so that large quantities of cells are obtained. The easiest way is, of course, to print directly with SVF, which is a mixture of all cell types.
In addition to the advantage of good access, SVF solves another problem: that the regulations on culturing cells that are intended to be returned to the patient are rigorous. The fact remains that this intended method means subjecting the tissue and cells to enzymatic treatment, but the method is nonetheless much more directly applicable than if the stem cells also have to be grown first.
Either the cells can be included directly in the printing process or they can be grown so that large quantities of cells are obtained. The easiest way is, of course, to print directly with SVF, which is a mixture of all cell types.
In addition to the advantage of good access, SVF solves another problem: that the regulations on culturing cells that are intended to be returned to the patient are rigorous. The fact remains that this intended method means subjecting the tissue and cells to enzymatic treatment, but the method is nonetheless much more directly applicable than if the stem cells also have to be grown first.
Blood vessels in 3D-bioprinted constructs
The single biggest limitation on the maximum size of constructs that should be printable is the transport of oxygen and nutrients to the cells in the printed tissue. Without blood vessels, the maximum thickness is about 0.5 mm — that is, twice the diffusion distance.
Work plan and preliminary results
Method 1
We did the printing with microfractured fat mixed in the bioink. This fat from liposuction is in tiny pieces that have undergone mechanical processing only. This means that all the fat components are included and the cells, such as stem cells, are therefore in their proper environment. In addition, fragments of blood vessels with their cells are also included. In one pilot project, we printed pieces 3 mm thick and implanted them in nude mice. After 30 and 60 days, the pieces proved to be living and the cells were thriving well, although the mixture was significantly thicker than would be possible. We could see blood vessels both on the edges and inside, in the middle of the pieces, and inside the blood vessels there were red blood cells. Thus, all of a sudden, we had created functioning blood vessels in a 3D- bioprinted construct.
This was a crucial breakthrough that has allowed us to continue working with thicker constructs and integrate vessels from the microfractured fat into the construction process. Below, there is an image showing how skin can attach to printed cartilage. This may conceivably be refined by printing cartilage centrally, surrounded by fat, and then putting skin on top of it. This means that we then genuinely have all the layers needed for an outer ear: cartilage, subcutaneous fat, and skin.
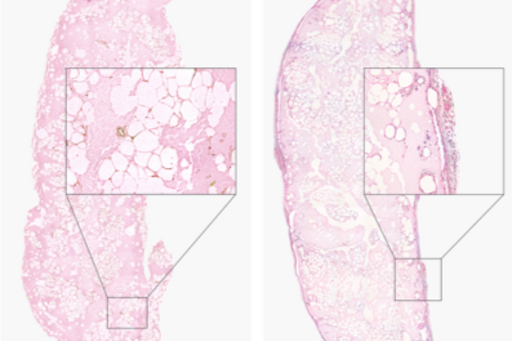
Above: 3D-bioprinted, microfractured fat after 7 and 30 days in vivo. The fat survived, despite its 3 mm thickness, which suggests a functioning microcirculation.
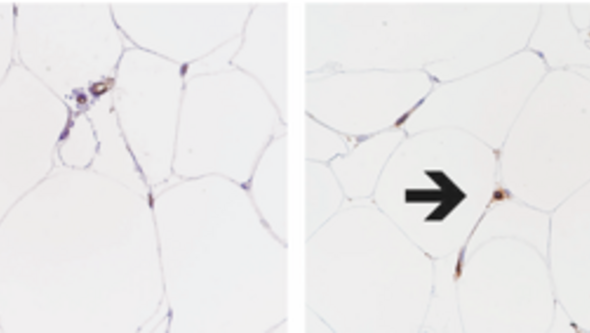
Above: the printed fat that contained capillary structures positive for anti-CD31 and stem cells positive for anti-CD 90.
Method 2
Another way to create vascular supply in 3D-bioprinted constructs is to print their tissue in porous form, as a three-dimensional mesh. Then the channels in the tissue would reduce the distance into the cells, compared with the construct being solid. So far, this reasoning has largely been theoretical, but we have succeeded in showing that tissue from the recipient can grow into the pores of the network, and that there are working blood vessels in this tissue. We have microscopic studies, and now also circulation studies with magnetic cameras, which show that the blood vessels work. This means that the oxygen supply to the center of a structure can be secured with the shape of the structure itself.
Method 3
We have just started working on a third way of influencing vascular formation. Since we can sort out, for example, vascular (endothelial) cells from adipose tissue, we want to try to create the vessels themselves more directly. It is already known that one can, for example, make endothelial cells adhere to surfaces that have been pretreated by covering the surface with proteins, such as fibronectin. We have now made endothelial cells from umbilical cord blood (HUVEC) attach to surfaces of our bioink (nanocellulose) that have been pretreated with fibronectin and other proteins. In the next step, which is currently under way, we have induced endothelial cells from our own cell source, the fat, to adhere. Similarly, the cell mixture in SVF shows excellent adherence. This means that we have tools to try to sow cells in channels inside the construct, thereby creating blood vessels.
Next stage
Summing up we thus have several different tools for trying to create vascular supply in the construct: printing in three-dimensional mesh structure; using microfractured fat, with the vessels forming spontaneously from the constituent fragments of blood vessels; and attempting to create vessels with endothelial cells. These methods can conceivably be combined in various ways, but it is all about creating circulation in the printed tissues so that the size is not limited by the diffusion distance.
3D-bioprinted skin
The skin is our largest organ, and two of its most important functions are protecting the body from the surroundings and preventing fluid loss. An injury to the skin that does not heal fast enough can have major consequences for the patient and result in death. Every year, approximately 11 million people around the world suffer from burns severe enough to require hospitalization (according to WHO). The degree (depth) of the burn plays a major part in the skin's capacity for healing. Burns of a higher degree result in nonfunctioning or nonexistent wound healing. In this case, the patient must rapidly get help to prevent fluid loss and infections that, at worst, may be fatal. Burns are treated by various means, including transplantation of the patient's own skin. When burns cover large parts of the body, the patient's own healthy skin cannot be used for treatment, and this is where new treatment methods are required to facilitate healing for the patient.
The purpose of our project is to use 3D-bioprinting technology to print "skin" from the patient's own cells, along with supporting material. These constructs are then transplanted onto the patient, whose body acts as the ultimate incubator for optimal cell growth and new skin formation. The innovative aspect of this idea is the ability to design patient-specific grafts (3D-bioprinted constructs) in clinically approved materials without using large areas of the patient's own skin. This would mean less pain for the patient, since there would be no need to remove large pieces of skin but improved healing would nonetheless take place, in that the grafts resemble complete skin.
Plan of work
For bioink development, fibrin and collagen will be used as the main ingredients and mixed with cells or microscopic pieces of skin. Evaluation will be done by looking at rheological properties, printability, and cytotoxicity. 3D-bioprinted skin constructs will be grown in vitro and used in two animal models, nude mouse and pig, in which we plan to study how the printed skin develops and how the wounds heal.
In the in vitro models, we can evaluate cell viability, collagen production and other skin-specific markers. Printed skin will also be placed in wounds punched in human skin that is excess tissue from plastic surgery. In these created wounds, tissue regeneration can be studied in a reproducible manner.
For nude mice, 3D-bioprinted tissue that contains human cells can be transplanted without the tissue being rejected.
Porcine wound healing most resembles that of humans. The skin of pigs is structured in the same way as in humans, and heals in a similar way. In addition, pigs are immunospecific individuals, just like humans.
Preliminary results
Printability
The printability of the ink is affected by the quantities of alginate and fibrin it contains. Both bioinks based on cellulose show good printability.
Collagen is a more fluid material, and another printing method needs to be applied. This method, known as FRESH (freeform reversible embedding of suspended hydrogels), involves printing in a support structure of gelatin. The gelatin is then removed at the same time as the collagen is crosslinked and its 3D structure obtained.
Cell survival
In vitro experiments with nanocellulose ink and fibrin ink (nanocellulose, alginate, and fibrin) have been performed. Fibroblasts were mixed with the various bioinks and printed for skin constructs. These were then transplanted into a wound model of human skin and monitored up to 28 days. Cell viability was high throughout the experiment and the fibrin ink showed stretched fibroblasts as early as day 1. Stretched cells are a sign that the cells have bound to the surface and assumed a more tissue-like morphology. Keratinocytes were seeded on the construct after 14 days and then allowed to differentiate in air. Histological staining was done to examine the morphology of the cells and possible collagen production. The sections show many cells at the surface of the construct, and there was some collagen production among these cells. In the middle of the construct, however, the cells are of a rounder shape and no positive staining for collagen was visible.
Transplant for wound model
To evaluate 3D bioprinting as a method of facilitating wound healing, a model consisting of normal healthy human skin was used. Experimental wounds (3 mm diameter) were punched in the skin. Constructs consisting of various types of ink mixed with human fibroblasts were transplanted into the wounds. Experiments were also performed with a separate layer of keratinocytes on top of the constructs. The wound model was monitored over time to study the healing process and cell viability in the construct. The results indicate that the cells survive the process and are viable in the healing wounds. No problems were identified in terms of biocompatibility of the constructs.
In vivo
To study the 3D-bioprinted skin in a more complex environment, animal model experiments were done. The current ‘nude mouse’ model was chosen because of these animals’ lack of a normal immune system. This allows transplantation of human cells without rejection reaction.
Constructs consisting of various inks have been 3D-bioprinted, along with human fibroblasts and keratinocytes, were placed under the skin of nude mice. After 14 and 30 days, the constructs were removed, fixed, and embedded in paraffin. Tissue sections from the various constructs were stained using immunohistochemistry to detect the presence of mature keratinocytes, proliferative cells, and blood vessels. Viable cells were identifiable in and around the constructs at all times. The presence of blood vessels was demonstrated by staining of the von Willebrand factor protein. Vascular growth is fundamental for long-term survival of the cells in the constructs and their tissue integration.
In box below: CK14-positive keratinocytes surrounding 3D-bioprinted skin
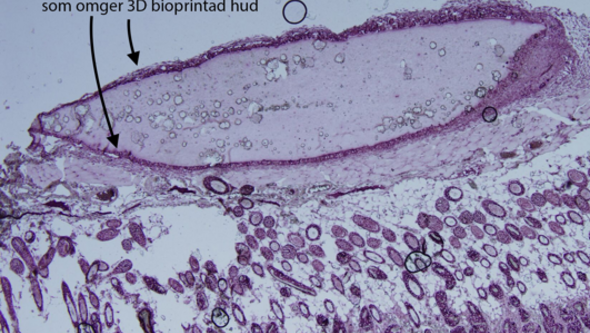
Figure 6. The image above shows a piece of 3D-bioprinted skin that has been in vivo for 30 days. Around the edges are CK14-positive cells — that is, the skin cells keratinocytes, which appear to be thriving and are proliferating.
Next stage
As the next stage in the sequence of planned experiments, we will use collagen ink, possibly in combination with fibrin to further stimulate the fibroblasts to grow.
As for cells, the next step is to mix SVF with stem cells in the skin printing to see if the growth of fibroblasts and keratinocytes improves.