 Arsenic and cadmium are two toxic elements for humans and they occur naturally in bedrock. Arsenic can poison groundwater, resulting in local populations in some places in the world being continually exposed.
Arsenic and cadmium are two toxic elements for humans and they occur naturally in bedrock. Arsenic can poison groundwater, resulting in local populations in some places in the world being continually exposed.
“Some crops, particularly rice, absorb arsenic very effectively because the proteins in the roots cannot distinguish between arsenic and the essential element phosphorus,” says Therese Jacobson, doctoral student at the Department of Chemistry and Molecular Biology. “Cadmium, in turn, is spread mainly by fertilizers and cigarette smoke.”
Her doctoral thesis deals with research trying to understand how cells respond to and defend against arsenic and cadmium. Therese and her colleagues have succeeded in identifying a defence mechanism in yeast cells. The mechanism involves the secretion of a type of molecule, glutathione, which consists of three amino acids and which binds arsenic outside the cells.
“By binding the arsenic, it is prevented from being absorbed into the cells,” she says.
Contribute to proteins clumping together
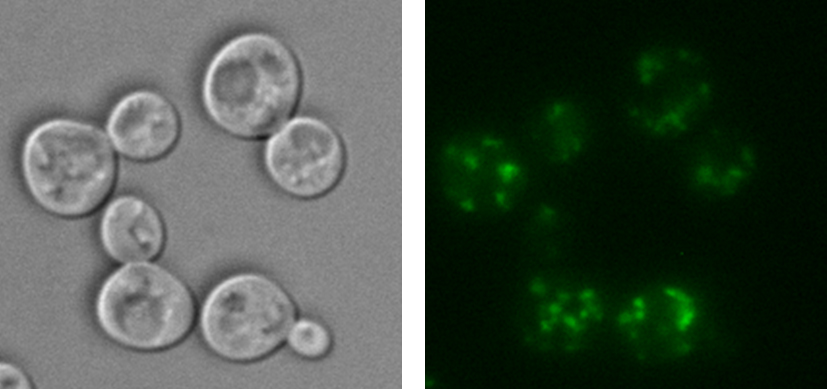
 Therese has concluded in her research that arsenic and cadmium contribute to proteins clumping together and forming aggregates. This aggregation, in turn, is negative for the cells — several neurodegenerative diseases, like Alzheimer's and Parkinson's, are linked to proteins forming aggregates. Therese’s research shows that arsenic and cadmium contribute to protein aggregation in different ways.
Therese has concluded in her research that arsenic and cadmium contribute to proteins clumping together and forming aggregates. This aggregation, in turn, is negative for the cells — several neurodegenerative diseases, like Alzheimer's and Parkinson's, are linked to proteins forming aggregates. Therese’s research shows that arsenic and cadmium contribute to protein aggregation in different ways.
“Arsenic prevents chaperone proteins from performing their important function,” she says. “Chaperones help newly formed proteins to take their correct three-dimensional shape. If these newly-formed proteins do not take their proper shape, this increases the likelihood that they will clump together with other proteins to form aggregates.”
Cadmium, in turn, affects zinc-binding proteins. Zinc ions are important for certain proteins to take their proper shape, and this research shows that cadmium appears to replace the zinc in these proteins, thus leading to aggregation.
Contact
Therese Jacobson, the Department of Chemistry and Molecular Biology
therese.jacobson@gu.se, +46 (0)31-786 2588, +46 (0)736-381060
Read more
Cellular Responses to Arsenite and Cadmium - Mechanisms of Toxicity and Defense in Saccharomyces cerevisiae
 Arsenic and cadmium are two toxic elements for humans and they occur naturally in bedrock. Arsenic can poison groundwater, resulting in local populations in some places in the world being continually exposed.
Arsenic and cadmium are two toxic elements for humans and they occur naturally in bedrock. Arsenic can poison groundwater, resulting in local populations in some places in the world being continually exposed. Therese has concluded in her research that arsenic and cadmium contribute to proteins clumping together and forming aggregates. This aggregation, in turn, is negative for the cells — several neurodegenerative diseases, like Alzheimer's and Parkinson's, are linked to proteins forming aggregates. Therese’s research shows that arsenic and cadmium contribute to protein aggregation in different ways.
Therese has concluded in her research that arsenic and cadmium contribute to proteins clumping together and forming aggregates. This aggregation, in turn, is negative for the cells — several neurodegenerative diseases, like Alzheimer's and Parkinson's, are linked to proteins forming aggregates. Therese’s research shows that arsenic and cadmium contribute to protein aggregation in different ways.