Trobos Group
Short description
The Trobos Group is led by Associate Professor Margarita Trobos and has a research focus on biomaterial-associated infections, covering aspects on the pathogenesis (biofilm mechanisms and antimicrobial resistance) and evaluating novel diagnostic, preventive and therapeutic strategies
Research field
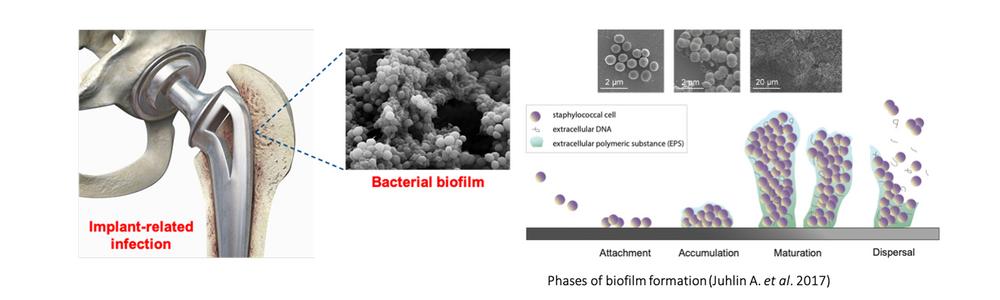
Infection on implants is one of the worst complications and numbers are increasing due to the large elderly population, representing more than half of hospital-acquired infections. These infections are caused by microorganisms that grow in biofilms and are difficult to diagnose and treat. The global rise on antibiotic resistance challenges their control and require urgent innovative alternatives to antibiotics.
Projects
1. Strategies against antibiotic resistant biofilms in orthopaedic device-related infection
Orthopaedic device-related infection (ODRI) leads to healthcare costs, suffering and mortality. Repeated surgeries and implant removal are often needed in the attempt to heal the infection. Approximately 37 000 primary hip and knee arthroplasty procedures are performed annually in Sweden. Periprosthetic joint infections (PJI) occur in 1-3 % of patients undergoing hip arthroplasty surgery. The infection incidence is projected to raise due to the increasing elderly population and antimicrobial resistance.
The overall purpose of this 3-year multidisciplinary project is to increase the scientific knowledge of ODRI. The aims are to investigate aspects of the pathogenesis and evaluate novel diagnostics and treatments to improve patient outcomes and reduce antibiotic resistance.
The scientific approach consists of clinical, preclinical and basic research, implemented by researchers from Biomaterials (University of Gothenburg), Orthopaedics and Infectious Diseases (Sahlgrenska Hospital) and Bacterial Pathogenesis (Public University of Navarre). This collaboration will enable translational research to transfer preclinical results into clinical practice.
Publications:
- Morales-Laverde L, Trobos M, Echeverz M, Solano C, Lasa I. Functional analysis of intergenic regulatory regions of genes encoding surface adhesins in Staphylococcus aureus isolates from periprosthetic joint infections. Biofilm. Dec 2022;4:100093. DOI: 10.1016/j.bioflm.2022.100093.
- Morales-Laverde L, Echeverz M, Trobos M, Solano C, Lasa I. Experimental Polymorphism Survey in Intergenic Regions of the icaADBCR Locus in Staphylococcus aureus Isolates from Periprosthetic Joint Infections. Microorganisms. Mar 10 2022;10(3). DOI: 10.3390/microorganisms10030600
Period: 2022-2025
Funding: Swedish Research Council (VR) (2022-00853), the ALF agreement (ALFGBG-978896), the IngaBritt and Arne Lundberg Foundation (LU2021-0048), Handlanden Hjalmar Svensson Foundation, Adlerbertska Foundation and Doctor Felix Neubergh Foundation, Göteborgs Läkarsällskap/The Gothenburg Medical Society research grants (GLS-973592)
2. UTMOST – Ultra-thin monitoring sensors for implants
Although thousands of implant surgeries are performed every week, so far it has not been possible to detect undesirable events, such as implant loosening and inflammation at an early stage. The general purpose of this truly interdisciplinary research project is to determine the feasibility of detecting successful and adverse local tissue responses by implementing battery-free and wireless ultrathin pressure sensors at the tissue-implant interface.
The project will result in a necessary and groundbreaking diagnostic tool as it enables early detection at the normally inaccessible tissue-implant interface. Potential applications are wide-ranging, and the technology could significantly impact the patient situation and the healthcare system in terms of improved quality of life and reduced societal costs.
Period: 2025-2029
Funding: Swedish Research Council (VR): Interdisciplinary Research Environment Grants 2024
3. Virulence inhibitors for the treatment of soft tissue infections
In collaboration with industrial partners, the group is currently addressing alternative treatments for chronic wounds to stop bacterial communication and virulence.
The overall purpose of this research project is to create a deeper understanding of the role of bacterial colonisation and infection in chronic wounds, more specifically the impact of bacterial virulence to the pathogenicity will be studied. The possibility of using virulence inhibitors as a new treatment strategy for hard-to-heal wounds will be pre-clinically evaluated in vitro and in viv
Period: 2017-2022
Funding: Swedish Foundation for Strategic Research (SSF; RMA15-0110 2016), Mölnlycke Health Care AB (Sweden), European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 754412 (MoRE2020 - Region Västra Götaland), CARe - Centre for Antibiotic Resistance Research at University of Gothenburg, Handlanden Hjalmar Svensson Foundation, Adlerbertska Foundation and Doctor Felix Neubergh Foundation.
Publications:
- Gerner E, Giraldo-Osorno PM, Johansson Loo A, Firdaus R, Ben Amara H, Werthen M, Palmquist A, Thomsen P, Omar O, Almqvist S, Trobos M. Targeting Pseudomonas aeruginosa quorum sensing with sodium salicylate modulates immune responses in vitro and in vivo. Front Cell Infect Microbiol. 2023;13:1183959
- Turner AB, Gerner E, Firdaus R, Echeverz M, Werthén M, Thomsen P, Almqvist S, Trobos M. Role of sodium salicylate in Staphylococcus aureus quorum sensing, virulence, biofilm formation and antimicrobial susceptibility. Front Microbiol. 2022;13:931839. DOI: 10.3389/fmicb.2022.931839
- Gerner E, Almqvist S, Thomsen P, Werthen M, Trobos M. Sodium Salicylate Influences the Pseudomonas aeruginosa Biofilm Structure and Susceptibility Towards Silver. Int J Mol Sci. Jan 21 2021;22(3)DOI:10.3390/ijms22031060.
- Gerner E, Almqvist S, Werthen M, Trobos M. Sodium salicylate interferes with quorum-sensing-regulated virulence in chronic wound isolates of Pseudomonas aeruginosa in simulated wound fluid. J Med Microbiol. Apr 22 2020;DOI:10.1099/jmm.0.001188.
4. Treatment of periprosthetic joint infections guided by Minimum Biofilm Eradication Concentration (MBEC) in addition to Minimum Inhibitory Concentration (MIC)
A new diagnostic method is under evaluation to guide treatment decisions of orthopaedic infections caused by biofilms. Staphylococci account for most deep infections associated with orthopaedic device related infections. It is important to investigate if these infections have a biofilm origin and to determine the biofilm antimicrobial susceptibility to improve treatment strategies. Retrospective and prospective clinical studies will evaluate the clinical relevance and implementation of the diagnostic method, in collaboration with the clinical departments of Orthopaedics and Infectious Diseases.
Period: 2017-2023
Funding: Swedish research council (VR, 2020-05703), ALF agreement (ALFGBG-725641; ALFGBG-719961), European Union Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754412 (MoRE2020 - Region Västra Götaland), CARe - Centre for Antibiotic Resistance Research at University of Gothenburg, the IngaBritt and Arne Lundberg Foundation (LU2021-0048), Handlanden Hjalmar Svensson Foundation, Adlerbertska Foundation, Doctor Felix Neubergh Foundation, Göteborgs Läkarsällskap/The Gothenburg Medical Society research grants (for PhD-studies and Svea Bäcksins grant GLS-780551, GLS-973592).
Publications:
- Tillander JAN, Rilby K, Svensson Malchau K, Skovbjerg S, Lindberg E, Rolfson O, Trobos M. Treatment of periprosthetic joint infections guided by minimum biofilm eradication concentration (MBEC) in addition to minimum inhibitory concentration (MIC): protocol for a prospective randomised clinical trial. BMJ Open. Sep 15 2022;12(9):e058168. DOI: 10.1136/bmjopen-2021-058168.
- Trobos M, Firdaus R, Svensson Malchau K, Tillander J, Arnellos D, Rolfson O, Thomsen P, Lasa I. Genomics of Staphylococcus aureus and Staphylococcus epidermidis from Periprosthetic Joint Infections and Correlation to Clinical Outcome. Microbiology Spectrum. Jun 28 2022:e0218121. DOI: 10.1128/spectrum.02181-21
- Zaborowska M, Tillander J, Branemark R, Hagberg L, Thomsen P, Trobos M. Biofilm formation and antimicrobial susceptibility of staphylococci and enterococci from osteomyelitis associated with percutaneous orthopaedic implants. J Biomed Mater Res B Appl Biomater. Nov 2017;105(8):2630-2640. DOI:10.1002/jbm.b.33803.
- Svensson Malchau, K., Tillander, J., Zaborowska, M., Hoffman, M., Lasa, I., Thomsen, P., Malchau, H., Rolfson, O. & Trobos, M. Biofilm properties in relation to treatment outcome in patients with first-time periprosthetic hip or knee joint infection. J Orthop Translat 30, 31-40 (2021).
5. BIOREMIA – European Training Network: Biofilm-resistant materials for hard tissue implant applications
BIOREMIA are research and educational training programmes to develop biofilm-resistant materials for bone implant applications. BIOREMIA aims to improve the patient quality of life by minimizing infection rates of medical implants. Innovative material-based solutions with enhanced antibacterial and antifouling functionality will be developed for improved biological acceptance of implants for bone-related applications (orthopaedics and dentistry).
On 30 June 2024 the BIOREMIA training network came to its end. The results of the ESRs’ PhD research work are highlighted in the numerous publications and participation in international conferences. Find out more in the BIOREMIA dissemination section.
https://www.bioremia.eu/dissemination
Period: 2020-2024
Funding: 4-year project funded by the European Commission under Horizon 2020 Marie Skłodowska Curie Actions.
Website: www.bioremia.eu
Publications:
- Turner, A.B., Zermeño-Pérez, D., Mysior, M.M., Giraldo-Osorno, P.M., O’ Gorman, E., Oubihi, S., Simpson, J.C., Lasa, I., Ó Cróinín, T., Trobos, M. Biofilm morphology and antibiotic susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) on poly-D,L-lactide-co-poly(ethylene glycol) (PDLLA-PEG) coated titanium. Biofilm, Volume 8, 2024,100228. ISSN 2590-2075.
https://doi.org/10.1016/j.bioflm.2024.100228. - Giraldo-Osorno PM, Turner AB, Mollet Barros S, Büscher R, Guttau S, Asa’ad F, Trobos M, Palmquist A. Anodized Ti6Al4V-ELI, electroplated with copper is bactericidal against Staphylococcus aureus and enhances macrophage phagocytosis. Journal of Materials Science: Materials in Medicine. Accepted.
- A.B. Turner, P.M. Giraldo-Osorno, Y. Douest, L.A. Morales-Laverde, C.A. Bokinge, F. Asa'ad, N. Courtois, A. Palmquist, M. Trobos, Race for the surface between THP-1 macrophages and Staphylococcus aureus on various titanium implants with well-defined topography and wettability, Acta Biomater 191 (2025) 113-139.
- P.M. Giraldo-Osorno, K. Wirsig, F. Asa'ad, O. Omar, M. Trobos, A. Bernhardt, A. Palmquist, Macrophage-to-osteocyte communication: Impact in a 3D in vitro implant-associated infection model, Acta Biomater 186 (2024) 141-155.
- Turner AB, Gerner E, Firdaus R, Echeverz M, Werthén M, Thomsen P, Almqvist S, Trobos M. Role of sodium salicylate in Staphylococcus aureus quorum sensing, virulence, biofilm formation and antimicrobial susceptibility. Front Microbiol. 2022;13:931839. DOI: 10.3389/fmicb.2022.931839.
- Gerner E, Giraldo-Osorno PM, Johansson Loo A, Firdaus R, Ben Amara H, Werthen M, Palmquist A, Thomsen P, Omar O, Almqvist S, Trobos M. Targeting Pseudomonas aeruginosa quorum sensing with sodium salicylate modulates immune responses in vitro and in vivo. Front Cell Infect Microbiol. 2023;13:1183959. DOI: 10.3389/fcimb.2023.1183959.
- Akman A, Alberta LA, Giraldo-Osorno PM, Turner AB, Hantusch M, Palmquist A, Trobos M, Calin M, Gebert A. Effect of minor gallium addition on corrosion, passivity, and antibacterial behaviour of novel β-type Ti–Nb alloys. Journal of Materials Research and Technology. 2023 Jul 1;25:4110–24. DOI:10.1016/j.jmrt.2023.06.219.
- Bartkowska A, Turner AB, Blanquer A, Nicolenco A, Trobos M, Nogues C, Pellicer E, Sort J. Accelerated biodegradation of FeMn porous alloy coated with ZnO: Effect on cytocompatibility and antibiofilm properties. Surface and Coatings Technology. 2023 Oct 25;471:129886. DOI: 10.1016/j.surfcoat.2023.129886.
6. Joint program Chalmers–GU on biomaterial-associated infection
This is a multidisciplinary and twinning collaboration program between the department of Chemistry and Chemical Engineering (Chalmers University of Technology) and the department of Biomaterials (University of Gothenburg) on biomaterial-associated infections and their role in antimicrobial resistance. The knowledge generated can provide future therapeutic alternatives, strategies for infection control, and ultimately a decreased incidence of biomaterial-associated infections and most importantly find measures to fight antimicrobial resistance. The goal is to generate high quality scientific knowledge and to enable bedside translation of knowledge.
Period: 2020-2022
Funding: The Area of Advanced Materials of Chalmers and GU Biomaterials within the Strategic Research Area initiative launched by the Swedish Government.
Publications:
- Atefyekta S, Blomstrand E, Rajasekharan AK, Svensson S, Trobos M, Hong J, Webster TJ, Thomsen P, Andersson M. Antimicrobial Peptide-Functionalized Mesoporous Hydrogels. ACS Biomater Sci Eng. 2021. DOI: 10.1021/acsbiomaterials.1c00029.
7. Microbiological profile of bone-anchored hearing systems (BAHS)
The bone-anchored hearing system (BAHS) has evolved to a common treatment option for various types of hearing revalidation. The BAHS consists of an implant in the skull that breeches the skin. Soft tissue reactions are a common complication associated with BAHS and are generally poorly understood. This project includes prospective clinical trials and aims at investigating the influence of relevant bacteria for BAHS-associated inflammation, as well as evaluating abutments with different topologies and preventive measures with respect to the clinical outcome and the microbiological profile.
Different sampling methods (retrieval of abutment, collection of peri-abutment exudate using paper-points, and a small peri-abutment soft-tissue biopsy) have been tested for the identification and quantification of colonising bacteria around BAHS.
Period: 2017-2021
Funding: Oticon, Handlanden Hjalmar Svensson Foundation, Adlerbertska Foundation, Doctor Felix Neubergh Foundation.
Publications:
- Johansson ML, Omar O, Trobos M, Jonhede S, Peters H, Hultcrantz M, Thomsen P. Non-invasive sampling procedure revealing the molecular events at different abutments of bone-anchored hearing systems-A prospective clinical pilot study. Front Neurosci. 2022;16:1058689. DOI:10.3389/fnins.2022.1058689.
- Johansson ML, Calon TGA, Omar O, Shah FA, Trobos M, Thomsen P, Stokroos R, Palmquist A. Multimodal analysis of the tissue response to a bone-anchored hearing implant: presentation of a two-year case report of a patient with recurrent pain, inflammation, and infection, including a systematic literature review. Front Cell Infect Microbiol. 2021. DOI:10.3389/fcimb.2021.640899.
- Trobos M, Johansson ML, Jonhede S, Peters H, Hoffman M, Omar O, Thomsen P, Hultcrantz M. The clinical outcome and microbiological profile of bone-anchored hearing systems (BAHS) with different abutment topographies: a prospective pilot study. Eur Arch Otorhinolaryngol. Jun 2018;275(6):1395-1408. DOI:10.1007/s00405-018-4946-z.
- Calon TGA, Trobos M, Johansson ML, van Tongeren J, van der Lugt-Degen M, Janssen AML, Savelkoul PHM, Stokroos RJ, Budding AE. Microbiome on the Bone-Anchored Hearing System: A Prospective Study. Front Microbiol. 2019;10:799. DOI:10.3389/fmicb.2019.00799.
8. Barrier function and oral plaque formation on PTFE membranes for GBR
This project evaluates biofilm formation and barrier function against oral bacteria by nonresorbable polytetrafluoroethylene (PTFE) guided bone regeneration membranes having expanded (e-PTFE) and dense (d-PTFE) microstructure.
Funding: BIOMATCELL VINN Excellence Center of Biomaterials and Cell Therapy, the Swedish Governmental Agency for Innovation Systems (grant No 2018-00252), Innovationsfonden (Region Västra Götaland), the Osteology Foundation, Neoss Ltd, Handlanden Hjalmar Svensson Foundation, the Adlerbertska Foundation, the Doctor Felix Neubergh Foundation.
Publications:
- Turri, O. Omar, M. Trobos, P. Thomsen, C. Dahlin, Modulation of gene expression and bone formation by expanded and dense polytetrafluoroethylene membranes during guided bone regeneration: An experimental study, Clin Implant Dent Relat Res 26(2) (2024) 266-280.Turri A, Omar O, Trobos M, Thomsen P, Dahlin C. Modulation of gene expression and bone formation by expanded and dense polytetrafluoroethylene membranes during guided bone regeneration: An experimental study. Clin Implant Dent Relat Res. Jun 25 2023. DOI: 10.1111/cid.13241.
- Turri A, Cirgic E, Shah FA, Hoffman M, Omar O, Dahlin C, Trobos M. Early plaque formation on PTFE membranes with expanded or dense surface structures applied in the oral cavity of human volunteers. Clin Exp Dent Res. Nov 9 2020;DOI:10.1002/cre2.344.
- Trobos M, Juhlin A, Shah FA, Hoffman M, Sahlin H, Dahlin C. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (PTFE) membranes for guided bone regeneration. Clin Implant Dent Relat Res. 2018 Oct;20(5):738-748. doi: 10.1111/cid.12629. Epub 2018 Jul 24. PMID: 30039909.
9. SHIELD-Strategies for Healing Implant-associated infections and Enhancing Longevity in Devices.
The research focuses on implants used in orthopaedics, otology, and dentistry—areas where infections can cause serious complications and lead to costly revision surgeries.
Period: 2025-2029
Funding: The project is funded through the Marie Skłodowska-Curie Doctoral Networks, a prestigious EU initiative aimed at promoting cutting-edge research and international collaboration. In total, 27 partners in eleven countries are involved, including universities, companies, research institutes, and hospitals. It's based at the Institute of Clinical Sciences has been awarded €4,725,090 (approximately SEK 52 million) from the EU’s Horizon Europe program.
Margarita Trobos is the coordinator of the project and Liliana Andrea Morales Laverde is co-project manager
Read more about SHIELD in the news articles - Prestigious EU grant awarded to research on implant infections
Microbiology
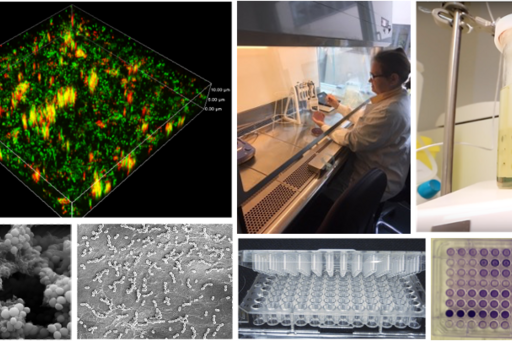
The unit for microbiology consists of a biosafety level 2 microbiology laboratory (L-verksamhet with GMM-use) and equipment such as CO2- and shaking-incubators, biofilm reactors, confocal microscope with incubation unit, NGS sequencer (MiSeq), plate reader, flow cytometry/cell sorter, ÄKTA flux, ultracentrifuge, Nanoparticle Tracking Analysis, and biobank (clinical and laboratory bacterial strains, bacterial DNA, bacterial RNA and bacterial extracellular vesicles).
The Department of Biomaterials have additional units for eukaryotic cell culturing (NucleoCounter and multiplex ELISA Q-View Imager), molecular biology (TissueLyser, QIAcube robot, qPCR, ddPCR, NGS sequencer, and Western Blot), histology and histomorphometry, chemistry (Tensiometer), and microscopy/imaging (Light/fluorescence microscopes, Confocal, Raman, AFM, micro-CT scan, and ChemiDoc).
Guest Researchers
2023:
Gabriela Paula Di Santo, Postdoctoral researcher, Department of Chemistry and Molecular Biology, University of Gothenburg, Sweden
Juan José Londoño Rueda, PhD student, PX Services SA (PXS), Switzerland
Tim Kreuz, PhD student, University of Cambridge (UCAM), United Kingdom
David Zermeño Pérez, PhD student, Ashland Specialties Ireland Ltd. (Ashland), Ireland
2022:
Sebastião Mollet Barros, PhD student, Stryker Trauma GmbH (Stryker), Germany
Aleksandra Bartkowska, PhD student, Universitat Autònoma de Barcelona (UAB), Spain
Yohan Douest, PhD student, MATEIS (INSA-Lyon) & Anthogyr SAS, France
John Michael Ahmed Escobar Hernandez, PhD student, Universitat Autònoma de Barcelona (UAB), Spain
Liliana Morales Laverde, PhD student, Microbial Pathogenesis Research Unit, NAVARRABIOMED and Public University of Navarre, Spain
Carl Anton Bokinge, Medical Doctor, “try-on-reach” program, University of Gothenburg, Sweden
2015:
Carles Taulé, Master student, Erasmus exchange student, University of Barcelona, Spain
Annika Juhlin, Master student, Chalmers University of Technology, Sweden
2013:
Annika Juhlin, Whitaker exchange student, University of Washington, USA
2012:
Magnus Forsberg, Master student, University of Gothenburg, Sweden



Previous Researchers
Adam Turner
PhD student at Department of Biomaterials
Period: Aug 2020-Sep 2024
Paula Giraldo
PhD student at Department of Biomaterials
Period: Aug 2020-Sep 2024
Rininta Firdaus
Postdoc at Department of Biomaterials
Period: Jul 2020-Jun 2023
Erik Gerner
PhD student at Department of Biomaterials
Period: Sep 2017-Sep 2022


