- Home
- Research
- Find research
- Dorota Raj - Signaling pathways and novel genetic factors involved in modulation of cisplatin response in C. elegans
Dorota Raj - Signaling pathways and novel genetic factors involved in modulation of cisplatin response in C. elegans
Dorota Raj defended her thesis on December 3, 2021

Modeling of cisplatin resistance in C. elegans to improve cisplatin cancer therapy
Cancer is a major cause of mortality throughout the world. Despite the effort of the scientific community, plenty remains to understand about the biology or genetics of cancer. Solid tumors contain both rapidly dividing and non-dividing cells whereas non-dividing cells can account for as much as 90-99 percent of the tumor mass. Therefore, it is challenging to fully understand the therapeutic effect of the drug using in vivo models where cells grow rapidly. And the solid tumors make up over 90 percent of all malignancies
– Cisplatin, a platinum-based chemotherapeutic, is one of the most effective chemotherapeutic drugs against cancer and it is widely used for treatment of solid tumors.
However, its efficiency is hampered by the ability of tumors to develop resistance against cisplatin (1), says Dorota Raj, with a Bachelor’s degree in Biotechnology at the University of Agriculture in Krakow, Poland and a Master’s degree in Biomedicine at Skövde University, Sweden (*).
Cisplatin exposure leads to damage of non-dividing cells (2) and results in toxicity in tissues like sensory neurons, kidneys, and ear. This led to speculation that cisplatin must have mechanism of action other than targeting of DNA and alternative effects of cisplatin might play a role in good therapeutic response.
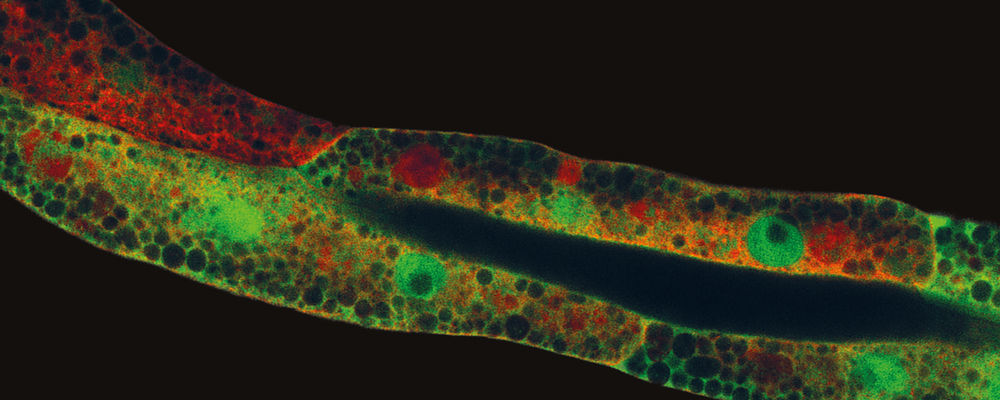
– The model organism used in my study is a roundworm C. elegans (3), which for molecular/cell biology analysis has great relevance to understand the mechanisms behind cisplatin resistance. C. elegans is naturally resistant to cisplatin treatment giving a great opportunity to model resistant tumors in vivo (living models). Therefore, using this nematode as a post-mitotic model we aimed to learn more about signaling pathways and genetic factors involved in the modulation of chemotherapeutic response on non-dividing cells.
For better understanding of cisplatin resistance
The main objective of this thesis was to have a better understanding of cellular pathways involved in response to cisplatin in order to address a major drawback of drug resistance caused by non-dividing cells.
This thesis builds on previous work, from the research group she is part of, showing that inactivation of ASNA-1 increases cisplatin sensitivity in C. elegans and mammalian cells.
The two major aims of her PhD project were to understand how ASNA-1 (4) mediates the response to cisplatin and what is the biochemical basis underlying the killing of post-mitotic tissues/cells by cisplatin.
– More specifically, we have discovered a new mechanism by which cisplatin-induced reactive oxygen species (ROS) (5) generation inactivates the cisplatin response function of ASNA-1 via its oxidation, which in turn perturbs the targeting of a special class protein called tail-anchored protein to the endoplasmic reticulum (ER) membrane. Cisplatin however had no effect on insulin secretion/signalling function of ASNA-1. This allowed us to separate clinically relevant ASNA-1 function in cisplatin sensitivity from insulin signaling. Next, analysis of tissue and genetic requirements of ASNA-1 allowed us to separate protein functions even further with a focus on growth, reproduction, and cisplatin response.
Lastly, they showed that the p38 MAPK (6) immunity pathway is required for cisplatin resistance and identified immune effectors as necessary for this response.
– In summary, using genetic and molecular analyses in C. elegans, we identified signaling pathways and novel genetic factors involved in the modulation of cisplatin response in post-mitotic cells with clear implications for strategies to refine and improve cisplatin cancer therapy.
* During her research position at the Sahlgrenska Center for Cancer Research at University of Gothenburg she became interested in chemotherapeutic research and pursued a career as PhD student in the same research group.
MORE FACTS
- Mechanism of resistance - There are two forms of resistance, innate resistance developed without any previous drug exposure and acquired resistance as an effect of drug exposure. The cisplatin resistance in dividing cells occurs via increased DNA repair, altered cellular accumulation and/or increased drug inactivation. The mechanism of resistance depends on the severity of resistance and those three mechanisms are not mutually exclusive. / Source page 17 in the dissertation
- Post-mitotic cells/non-dividing cells -– cell division is a process by which a parent cell divides into two or more daughter cells by a process called mitosis (Martin EA, Hine R (2020). A dictionary of biology (6th ed.). Oxford: Oxford University Press. ISBN 9780199204625. OCLC 176818780). A mature, terminally differentiated cell no longer able to undergo mitosis is defined as post-mitotic.
- C. elegans is a roundworm which exist in two forms: as self-fertilizing hermaphrodites and males. They are characterized by a short generation cycle of ~3.5 days which involves an embryonic stage, four larval stages, and an adult stage.
- ASNA-1 (ArSeNite-translocating ATPase family) is a protein involved in insulin secretion and cisplatin detoxification / Source the dissertation
- ROS (reactive oxygen species) - In a biological context, ROS are byproducts of the normal metabolism of oxygen. During times of environmental stress (e.g., UV or heat exposure), ROS levels can increase dramatically. This may result in significant damage to cell structures. / Source Wikipedia
- p38 MAPK pathway consists of mitogen-activated protein kinases (MAPKs) that are responsive to stress stimuli and infection. Source Wikipedia
Supervisor Gautam Kao
Co-supervisor: Peter Naredi, Lisa Nilsson and Johan Bourghardt Fagman
Opponent: Nektarios Tavernarakis, University of Crete, Heraklion, Greece
Examining Committee: Ingela Parmryd, Sun Nyunt Wai och Marc Pilon