SundenLab
Short description
In SundenLab, our focus lies in developing methods within synthetic organic chemistry to streamline and innovate synthesis processes. By creating innovative reactions for molecular assembly, we aim to provide chemists with new tools to make molecules. Our methods are not only directed towards cost-effectiveness and environmental friendliness but also open doors to the discovery of new pharmaceuticals and smart materials. By combining technical expertise with a sustainability perspective, we are shaping a future where synthesis processes are efficient, economical, and environmentally friendly.
Green chemistry
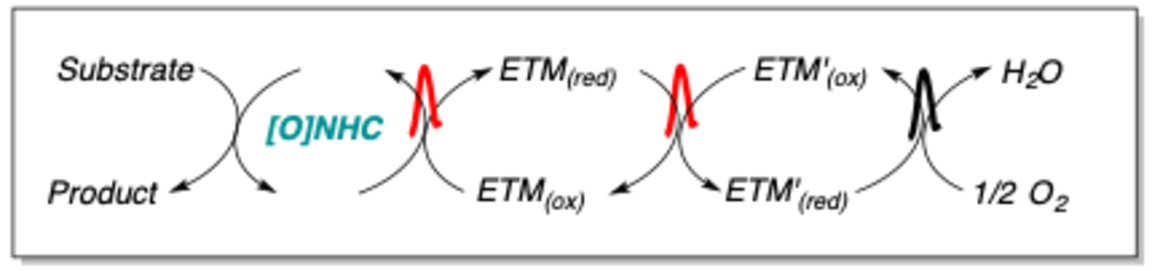
Oxidation reactions are of fundamental importance in organic synthesis. However, oxidation reactions still mainly rely on stoichiometric high molecular weight oxidants that are very often both hazardous and produces large amount of by-products. In the SundenLab we are interested in developing reactions that uses the oxygen content in air as the terminal oxidant. The result is a reaction that is without cost in oxidant and produces water as the sole by-product. We believe that most future oxidation reactions need to follow these design principles to be sustainable.
Publications
- Redox Active N-Heterocyclic Carbenes in Oxidative NHC Catalysis
- Aerobic Oxidative N-Heterocyclic Carbene Catalysis
- Organocatalytic valorisation of glycerol via a dual NHC-catalysed telescoped reaction
- Attractive aerobic access to the α,β-unsaturated acyl azolium intermediate: oxidative NHC catalysis via multistep electron transfer
Supramolecular chemistry
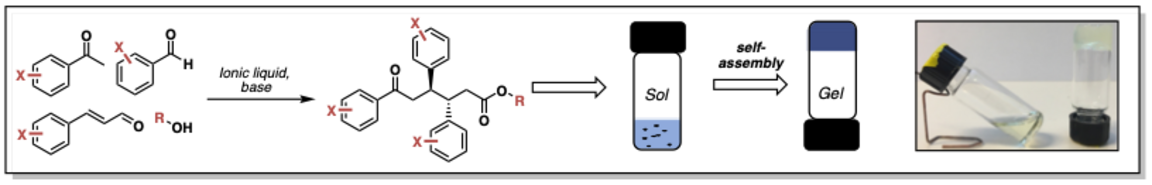
To accelerate the discovery and development of new low molecular weight compounds that can self-assemble into a supramolecular polymers or a gels the SundenLab started a research program in 2014 that aimed towards the development of multi-component synthesis strategies of gelators. In a multi-component reaction (MCR), 3 to 4 components are assembled into the desired molecule in only one synthetic step, usually under the formation of two or three new bonds. This one operation procedure permits to save time and money compared to classical LMWGs synthetic procedures. Providing that the reagents for such reaction are available, a high number of new compounds can be reached. For example: a three-component reaction where each of the components are available in 10 different forms will provide a collection of 1000 different compounds. MCRs has been used in the pharmaceutical industry for years to create molecular libraries but is rarely encountered in materials chemistry. Towards this end we developed multi-component synthesis of oxotriphenylhexanoate (OTHO) and has since then shown their ability to form gels in several different solvents including water. The OTHO MCR facilitates rapid screening, investigation, evaluation, and development of supramolecular materials with a desired molecular function and macromolecular function.
Publications
- Spatiotemporal Release of Singlet Oxygen in Low Molecular Weight Organo-Gels Upon Thermal or Photochemical External Stimuli
- Exploring Supramolecular Gels in Flow-Type Chemistry—Design and Preparation of Stationary Phases
- Writing and erasing multicolored information in diarylethene-based supramolecular gels
- Ionic Liquids as Precatalysts in the Highly Stereoselective Conjugate Addition of α,β-Unsaturated Aldehydes to Chalcones
Boron Chemistry

Aromatic compounds, endowed with diverse functionalities, serve as pivotal precursors in the synthesis of various compounds, particularly in medicinal applications. Traditional methods of aromatic compound functionalization with electrophiles frequently yield complex mixtures or necessitate intricate reaction conditions for selective tuning. However, harnessing the potential of boron, manifested in the form of BBr3, in conjunction with coordinating groups inherent to aromatic compounds, facilitates a selective functionalization approach.
In our research group, we are actively engaged in exploring transformations leveraging the heightened selectivity offered by borylation, thereby enabling selective secondary transformations. This strategic utilization of borylation presents a synthetic shortcut, markedly enhancing the efficiency and feasibility of several chemical processes. We have shown that it is possible to use this strategy to install halogens and phenyl groups in the ortho position to benzanilides and 2-phenyl pyridines.
Publications
- Stable BF2 Boracycles as Versatile Reagents for Selective Ortho C–H Functionalization
- Ortho Arylation of N-Aryl Amides and the Construction of Diagonal Tetraarylbenzenediamines and N-Doped Fulminenes via BBr3-Derived Dibromoboracycles
- Regioselective ortho halogenation of N-aryl amides and ureas via oxidative halodeboronation: harnessing boron reactivity for efficient C–halogen bond installation
- Site Selective Boron Directed Ortho Benzylation of N-Aryl Amides: Access to Structurally Diversified Dibenzoazepines
- Boron-Mediated Regioselective Aromatic C−H Functionalization via an Aryl BF2 Complex
Group members



