STandaRdizing Organs at risk volumes in radiatioN therapy planninG STRONG
Short description
STandaRdizing Organs at risk volumes in radiatioN therapy planninG – STRONG.
The research project aims to develop standardized, evidence-based and nationally established guidelines for defining organs at risk of radiation-induced toxicity in Swedish radiation therapy. Today, this is missing, which means that treatment decisions are made on varying evidence, which can lead to both reduced chances of curing the cancer disease as well as increased risks of side effects. The absence of a standard also makes comparisons of different treatment metrics difficult. Standardization promotes digital information exchange, which, for example, is needed for automated handling of medical images to support treatment planning. Our work will improve the knowledge about how to optimally use modern radiation therapy.
Background
Radiation therapy is used to cure the disease or relieve symptoms for approximately every second cancer patient and is one of the most advanced technological disciplines in health care. An important prerequisite for providing radiation therapy is that there is detailed information about the patient's tumor area and surrounding anatomy (organs at risk [OAR]) so that the treatment can be designed to protect healthy tissue while removing the cancer cells.
Today, there is no standardized way to define OAR:s in radiation therapy, which limits the understanding of relationships between radiation dose and side effects. In the absence of a common reference, the development of better treatment methods that could lead to reduced risks of side effects are limited since the interpretation of different treatment metrics between patients and clinics becomes unclear. Standardization of this area is also important to enable digital information exchange which, for instance, is needed to support automated image management in treatment planning.
Purpose
This project aims to develop guidelines for how to define OAR:s of different body regions by engaging Sweden's overall radiation oncology expertise to identify or create descriptions that are based on scientific evidence. Accepted guidelines will then be translated into a digital format using formal methods and semantic web technologies that also will be combined with advanced algorithm and software development.
Objective
Our goal is to contribute to the development of precision medicine in radiation therapy as evaluation of treatment effects will become safer and more individualized when standardized guidelines for OAR:s are used in treatment planning. Our work also promotes equal cancer care as quantitative measures of normal tissue doses can be compared to a same reference and thereby be better used to improve the quality of radiation therapy than what is possible today. We also hope that the work process of planning radiation therapy will benefit by our project also contributing to further the development of "auto-contouring". This means that advanced functionality in IT systems for treatment planning automatically searches for the anatomical shape of different OAR:s, which saves time for the staff and contributes to uniform risk organ volumes.
Results
For each body region / cancer diagnosis / clinically important OAR, the work is carried out in four steps: 1) guidelines for clinically important OARs are defined based on scientific evidence and community agreement; 2) differences between clinical and standardized OARs and impact on clinical decision making are evaluated; 3) guidelines for OARs based on standards in healthcare and image management are translated to allow for semantic interoperability and machine-readability; 4) anatomical accuracy of OARs by existing auto-contouring approaches and the proposed digital guidelines are investigated.
- Work for OARs of the male pelvic / prostate cancer began in 2019 and has resulted in two nationally established guidelines (Table 1; Figure 1; Scientific article 1; Degree project 1).
- Work for OAR:s in pediatric cancer treatments began in 2020 (Figure 2).
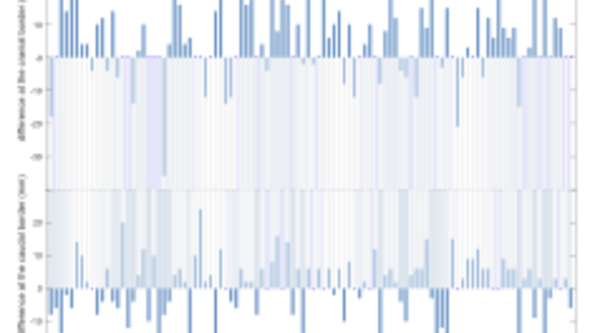
Figure 1
Differences in mm between clinical and standardized volumes for an organ at risk (rectum) for 102 patients treated for prostate cancer at Sahlgrenska University Hospital. A positive difference indicates that the clinical volume (R_Clin) is more cranially defined than the standardized volume according to the national guideline (R_Stand).
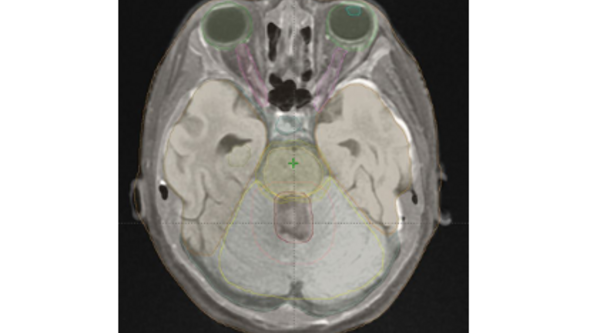
Figure 2
Organs at risk delineated on MRI data for a 10-year-old patient with medulloblastoma (treatment volumes in red / apricot). Green = eye; aqua = eye lens; pink = optic nerve; light blue = pituitary; brown=temporal lobe; olive = hippocampus; yellow = brain stem with safety margin; lemon = cerebellum.
Publications related to the project
Radiother Oncol, 2016 May;119(2):344-50
This projects has been approved by the Ethics Committee
Dnr: 641-17; T1115-18 och 2019-04207