Lightsheet 7
Light sheet fluorescence microscopy (LSFM) uses a thin sheet of light to excite only fluorophores within the focal volume. It is ideal for fast and gentle imaging of whole living model organisms, tissues and cells as they develop – over extended periods of time. Furthermore, the Lightsheet 7 is designed to handle large optically cleared specimens with subcellular resolution. Dedicated optics, sample chambers and holders allow adaption to the refractive index of your chosen clearing method.
The Lightsheet 7 Microscope
Light sheet fluorescence microscopy (LSFM)
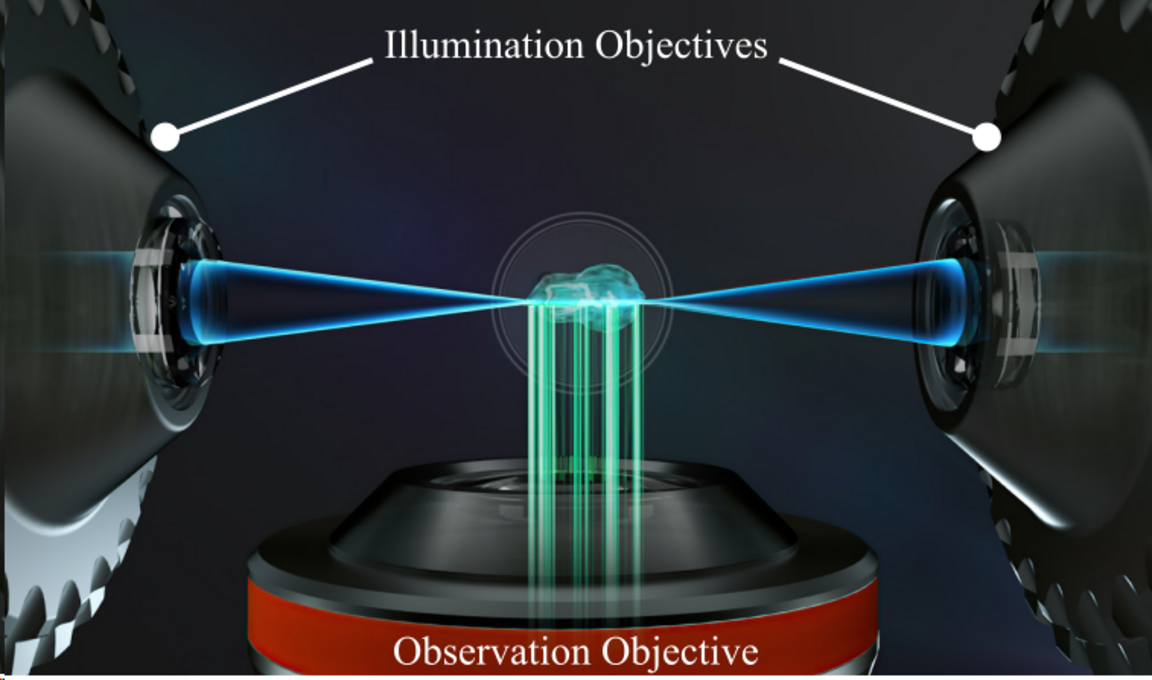
LSFM illuminates a thin slice of a fluorescent sample and observes the emission selectively created from this plane by using an objective placed perpendicularly to the incident light. As a consequence, the LSFM does not excite fluorophores and endogenous organic molecules outside the focal volume, in a conceptually similar way to a confocal microscope. The advantages of selective illumination become most apparent when complex and dynamic 3D structures are observed, ranging from specimens as large as cubic centimetres (e.g. embryos or cleared samples) to objects as small as organelles.

LS7 system characteristics
Illumination Optics
These are the objectives used to generate the incident lightsheet. The illumination optics can be adjusted to match the refractive index of the sample, being water (n=1.33) for live imaging or higher (n = 1.35 - 1.58) for optically cleared samples.
- LSFM 5X N.A. 0.1 foc
- LSFM 10X N.A. 0.2 foc
Detection Optics
These objectives are used to collect the fluorescence of the sample and create an image of the specimen on the detectors. They are selected in accordance to the illumination strategy (size of the light sheet).
Imaging with Water Immersion (refractive index 1.33)
- EC Plan-NEOFLUAR 5x / 0.16 N.A.
- W Plan-Apochromat 10x / 0.5 N.A.
- W Plan-Apochromat 20x / 1.0 N.A.
- W Plan-Apochromat 40x / 1.0 N.A.
Imaging with Clearing Immersion (refractive index 1.35-1.58)
- EC Plan-NEOFLUAR 5x / 0.16 N.A. + correction collar
- Clr Plan-APOCHROMAT 20x / 1.0 N.A. refractive index 1.35 - [1.38] - 1.41 (421459-9870-000)
- Clr Plan-NEOFLUAR 20x / 1.0 N.A. refractive index 1.42 - [1.45] - 1.48 (421459-9970-000)
- Clr Plan-NEOFLUAR 20x / 1.0 N.A. refractive index 1.48 - [1.53] - 1.58 (421459-9870-000)
Imaging with Translucence chamber for mesoscopy
- FLUAR 2.4 X / 0.12 N.A.
- EC Plan-NEOFLUAR 5x / 0.16 N.A. n=1.38
- EC Plan-NEOFLUAR 5x / 0.16 N.A. n=1.45
- EC Plan-NEOFLUAR 5x / 0.16 N.A. n=153

Light Sources
Lasers:
- 405 nm 50 mW
- 488 nm 50 mW
- 561 nm 50 mW
- 638 nm 75 mW
*Power: pre fiber
Transmitted LED
The microscope also has a LED array to observe the sample in transmitted mode. This allows easy manipulation of the sample within the imaging chamber.
Filters
Dichroic filter:
- LBF 405/488/561/640
Beam splitter for dual camera
- SBS LP 490
- SBS LP 560
- SBS LP 580
- SBS LP 640
Emission filters
- BP 420-470
- BP 505-545
- BP 575-615
Sample Chamber
The LS7 comes with specialized imaging chambers that adapt to the size of the specimen and the illumination + detection optics. We have the following chambers available:
- Chamber n=1.33 for water immersion
- Chamber large n=1.33-1.58 for large cleared specimens
- Chamber n=1.38-1.58 for high resolution cleared specimens
- Translucence chamber for mesoscopy (large[+] cleared specimens)

Cameras
Dual camera system
- 2 pco.edge 4.2 CLHS, sCMOS sensor, liquid cooling, aligned on a special C-mount for optimized image alignment on dual camera port
- Physical pixel size 6.5 microns
- Image pixel size (max) 1920x1920, 15 bit
- QE up to 82%
- Max. framerate 57fps at 1024 x 1024 in continuous z-drive mode
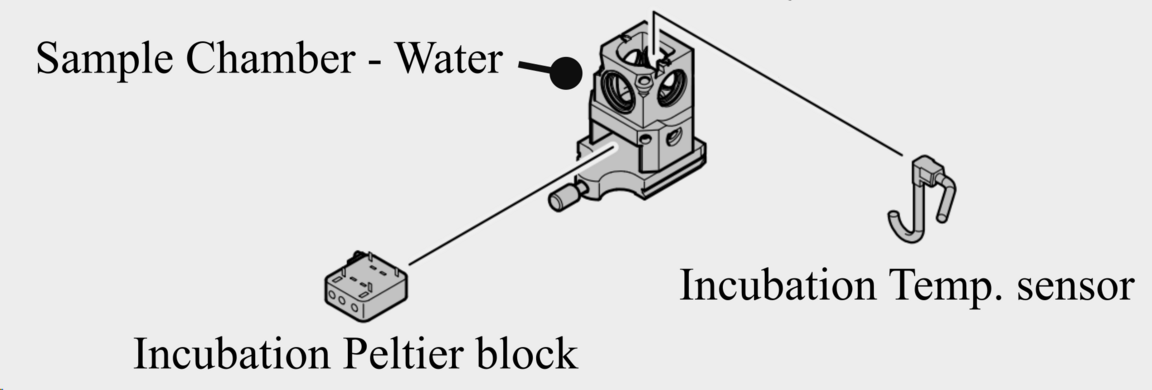
Incubation system for live imaging
Heating and cooling of the chamber via Peltier Block (10 - 42 C)
Temperature stability +- 0.1 C
CO2 module: 0 - 10 %
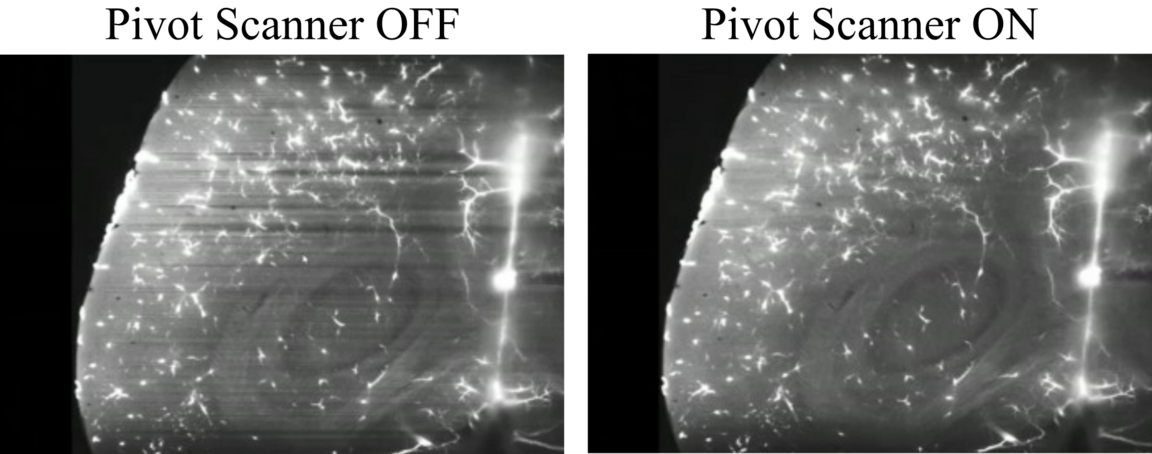
Pivot Scan
One of the drawbacks of the lightsheet illumination is the generation of shadows in the incident sheet when the beam encounters an obstacle. To compensate for this, a Pivot Scanner is used, where the lightsheet is "rotated" upwards and downwards. This way the shadows will be cast in different directions and averaged out.